Day 1 :
Keynote Forum
Vladimir P Torchilin
Director, Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University USA
Keynote: Combination siRNA/drug nanopreparations for multidrug resistant cancer
Time : TBA

Biography:
Vladimir P. Torchilin, is a University Distinguished Professor of Pharmaceutical Sciences and Director, Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston. His interests include drug delivery and targeting, nanomedicine, multifunctional and stimuli-sensitive pharmaceutical nanocarriers, biomedical polymers, experimental cancer therapy. He has published more than 400 original papers, more than 150 reviews and book chapters, wrote and edited 12 books, and holds more than 40 patents. Google Scholar shows more than 44,000 citations of his papers with H-index of 96. He is Editor-in-Chief of Current Drug Discovery Technologies, Drug Delivery, and OpenNano, Co-Editor of Current Pharmaceutical Biotechnology and on the Editorial Boards of many other journals. He received more than $30 M from the governmental and industrial sources in research funding. He has multiple honors and awards and in 2011, Times Higher Education ranked him number 2 among top world scientists in pharmacology for the period of 2000-2010.
Abstract:
Tumor therapy, especially in the case of multidrug resistant cancers, could be significantly enhanced by using siRNA down-regulating the production of proteins, which are involved in cancer cell resistance, such as Pgp or survivin. Even better response could be achieved is such siRNA could be delivered to tumors together with chemotherapeutic agent. This task is complicated by low stability of siRNA in biological surrounding. Thus, the delivery system should simultaneously protect siRNA from degradation. We have developed several types of lipid-core polymeric micelles based on PEG-phospholipid or PEI-phospholipid conjugates, which are biologically inert, demonstrate prolonged circulation in the blood and can firmly bind non-modified or reversibly-modified siRNA. Additionally, these nanopreparations can be loaded into their lipidic core with poorly water soluble chemotherapeuticagents, such as paclitaxel or camptothecin. In experiments with cancer cell monolayers, cancer cell 3D spheroids, and in animals with implanted tumors, it was shown that such co-loaded preparations can significantly down-regulate target proteins in cancer cells, enhance drugactivity, and reverse multidrug resistance.
Tumor therapy, especially in the case of multidrug resistant cancers, could be significantly enhanced by using siRNA down-regulating the production of proteins, which are involved in cancer cell resistance, such as Pgp or survivin. Even better response could be achieved is such siRNA could be delivered to tumors together with chemotherapeutic agent. This task is complicated by low stability of siRNA in biological surrounding. Thus, the delivery system should simultaneously protect siRNA from degradation. We have developed several types of lipid-core polymeric micelles based on PEG-phospholipid or PEI-phospholipid conjugates, which are biologically inert, demonstrate prolonged circulation in the blood and can firmly bind non-modified or reversibly-modified siRNA. Additionally, these nanopreparations can be loaded into their lipidic core with poorly water soluble chemotherapeutic agents, such as paclitaxel or camptothecin. In experiments with cancer cell monolayers, cancer cell 3D spheroids, and in animals with implanted tumors, it was shown that such co-loaded preparations can significantly down-regulate target proteins in cancer cells, enhance drug activity, and reverse multidrug resistance.
Keynote Forum
Alain L. Fymat
International Institute of Medicine and Science California, USA
Keynote: Drug Delivery Across the Blood Brain Barrier
Time : TBA

Biography:
Alain L. Fymat is a medical-physical scientist and an educator who was educated at the Universities of Bordeaux and Paris-Sorbonne, France, and the University of California at Los Angeles. He is the current President/CEO and Professor at the International Institute of Medicine & Science. He was formerly Professor of Radiology, Radiological Sciences, Radiation Medicine (Oncology), Critical Care Medicine, and Physics at several U.S. and European Universities. His current research interests lie at the interface between science and medicine (precision medicine, nanobiotechnology, nanomedicine, genetics/epigenetics/ecogenetics). He has extensively published (>> 300 scholarly publications) and lectured in several national and international academic, professional, governmental and industrial venues. He is a a Board member of several institutions, Editor of the Journals “Nanobiotechnology” and “Global Nanomedicine”, and Honored Editor of the Journals “Cancer Prevention and Current Research” and “Nanomedicine Research”.
Abstract:
There are approximately 400 known neural disorders some of which being due to a disruption or failure of the blood brain barrier (BBB) such as, for example: meningitis (an inflammation of the meninges or membranes surrounding the brain and spinal cord); epilepsy (chronic or acute seizures caused by inflammation); multiple sclerosis (MS - a disease of the immune system or/and the breaking down of the BBB in a section of the brain or spinal cord); Alzheimer disease (AD - a disease in which amyloid beta contained in blood plasma enter the brain and adhere to the surface of astrocytes); possibly prion and prion-like diseases such as Parkinson disease (PD) and AD; HIV encephalitis (a precursor of HIV-associated dementia in which latent HIV can cross the BBB inside circulating monocytes in the blood stream); and systemic inflammation (sterile or infectious) that may lead to effects on the brain, cause sickness behavior and induce or/and accelerate brain diseases such as MS and PD. There are currently active investigations into treatments for a compromised BBB. As a consequence of the growing aging population, many such neurodegenerative diseases, cancer and infections of the brain will become more prevalent. Of interest here are those disorders requiring treatment by delivery of drugs across the brain protective barriers.
I will review the difficulties inherent in the delivery of drugs across the BBB in the treatment oif the above neurological disorders, and discuss the mechanisms for drug targeting both “through” and “behind” the BBB. I will also suggest approaches for the enhancement of drug delivery including physiological approaches, chemical and biological delivery, disruption of the BBB system, the use of molecular Trojan horse systems, and the various nanoparticle and nano delivering devices.
Keynote Forum
Irach B. Taraporewala
Sitara Pharmaceutical Group, New York, USA
Keynote: Modes of Topical and Localized Drug Delivery of Nanoparticle and Microparticle Based Formulations
Time : TBA

Biography:
Irach B. Taraporewala is President of the Sitara Pharmaceutical Group. He is an innovator-serial entrepreneur; pharma executive, drug delivery & development expert provides consultant services for product development and strategy, Regulatory Affairs. Former CEO and President at OHR Pharmaceutical, he developed AVR118, a peptide immunomodulatory drug parenteral and topical formulations for cancer cachexia and squalamine eye drop formulations for treating retinal diseases, He has worked on drug-device combination devices for ophthalmic and intranasal drug delivery and development of molecular diagnostic products as well.
Abstract:
As the field of nanomedicine advances into multiple therapeutic areas, there is a concomitant need to develop novel strategies and methodologies for various modes of administration targeting different regions of the body. Local or topical modes of delivery to localize the drug action is an important aspect of treatments in dermatology, rheumatology, intranasally administered drugs, ocular drugs, drugs to localize in specific organs’ solid tumors and spinally administered drugs, for example. Drug delivery strategies are currently being developed for the efficient delivery of sustained release nanoparticle and microparticles through topical or local delivery of therapeutic agents by the subcutaneous, transdermal, intra-articular, ocular, inhalation, sublingual and intrathecal routes. The talk will focus on the guidelines for developing suitable formulations and delivery systems for these various routes of drug administration. Self-assembling nanoparticle drug delivery, development of nanoparticle patches, microneedle delivery, dissolving microneedle methodologies, magneto-electric approaches, transdermal polymer patches, spun silver nanoparticles, nanoparticle eluting stents, intrathecal delivery via polymeric nanocomposite hydrogels and development of intranasal formulations of nanoparticle based small molecule and macromolecule drugs will be the focus of the talk.

Localized drug delivery to internal ocular structures and to brain tissue, as in targeting brain tumors will also be addressed. Formulation strategies to lengthen residence time and provide longer sustained release in nanoparticle based formulations will be discussed, as well as regulatory considerations in the development of clinically viable nanomedicine-based drug products and diagnostic agents.
Keynote Forum
Stephen M Mahler
Australian Institute for Bioengineering and Nanotechnology, University of Queensland, Australia
Keynote: Antibody-targeted delivery of nanoparticles utilizing bispecific antibodies for applications in oncology
Time : TBA

Biography:
Stephen Mahler is a Senior Group Leader at the Australian Institute for Bioengineering and Nanotechnology and Director of the ARC Training Centre for Biopharmaceutical Innovation at the University of Queensland. His expertise is in the discovery, research and development of biologic medicines, particularly engineered monoclonal antibodies. His recent research endeavors include targeting nanoparticles using bispecific antibodies. He has a number of collaborations with key biotechnology companies in Australia and internationally, and was part of the team at Cambridge Antibody Technology, UK that developed the technology for the development of the TNF antagonist Humira, now the largest selling drug globally. He has particular interest in translating new biologics and nanomedicines to the clinic, and has been associated with a number of biologics entering clinical development.
Abstract:
Incorporating drugs such as small molecules, siRNA and proteins into nanoparticles seeks to achieve greater therapeutic efficacy and reduced systemic toxicity. However, despite extensive research into nanomedicine, there are few approved, passively-targeted products approved for use including liposomal nanoparticles Myocet, Doxil, Daunoxome and Depocyt, Abraxane (albumin-based nanoparticle) and Genexol-PM (micelle).
There are a number of barriers to successful nanoparticle-mediated drug delivery, including the reticular endothelial system, efficient extravasation, crossing the anatomical and physical barriers between endothelial and tumour cells, penetrating the tumour structure, endocytosis uptake and intracellular release of therapeutic agents. Active targeting of nanoparticles using antibodies or peptides can improve nanoparticle delivery by enhancing uptake into targeted tumour cells. While targeting has little or no impact on systemic delivery challenges, actively-targeted nanoparticles offer the advantage of having increased tumour cell uptake through receptor-mediated endocytosis, compared to passively-targeted nanoparticles.
We are investigating a range of different strategies to empower nanoparticles with targeting antibody fragments that are highly specific to tumour cell receptor targets such as epidermal growth factor receptor (EGFR) and other tumour-associated targets. The targeting of nanoparticles through the use of bispecific antibodies is described.
Keynote Forum
Volkmar Weissig
Midwestern University, USA
Keynote: DQAsome-based delivery of Phosphorothioate gapmer antisense oligonucleotides as therapy for Clostridium difficile
Time : TBA

Biography:
Volkmar Weissig is a Tenured Full Professor of Pharmacology and Chair of the Department of Pharmaceutical Sciences and Co-Director of the Nanomedicine Center of Excellence in Translational Cancer Research at Midwestern University Glendale, AZ, USA. Dr. Weissig holds 16 patents and he has published over 100 research papers, review articles and book chapters, mostly in the area of nanodrugdelivery systems. He also edited and published 8 books. Since the late 1990s he has pioneered the development of vesicular nanocarriers for the delivery of biologically active molecules to mitochondria within living mammalian cells in vitro and in vivo. In 2009 he was inducted into the World Technology Network as a Fellow and in 2014 Dr. Weissig was elected Inaugural President of the World Mitochondria Society.
Abstract:
Statement of the Problem: C. difficile infection (CDI) is a major healthcare burden due its prevalence, its communicable nature within healthcare settings, its frequent need for multiple rounds of conventional antibiotics and its predilection for severe forms of colitis.Unpredictable responses associated with conventional antibiotics have raised significant interest in designing alternative CDI therapies, among which “antisense antibiotics” (1) able to prevent the expression of bacterial genes through posttranscriptional mechanisms appeared of particular interest to us.

The purpose of this study was to test DQAsome (2-4) -like cationic nanovesicles (Figure 1, left) composed of bolaamphiphiles (Figure 1, right) as a delivery system for 2’-O-methyl phosphorothioate gapmer antisense oligonucleotides (ASO) in order to target the expression of essential genes of C. difficile. Methodology: The ASO were assessed for their ability to inhibit mRNA translation using luciferase reporter and C. difficile protein expression plasmid constructs in a coupled transcription-translation system. Bolasomes were prepared as described (2-4) and characterized by particle size distribution, zeta potential and oligonucleotide binding capacity. Anaerobic C. difficile log phase cultures were treated with serial doses of nanocomplexes obtained from incubating the cationic bolasomes with ASO’s. Results: Antisense gapmers for four chosen genetargets achieved nanomolar minimum inhibitory concentrations for C. difficile. No inhibition of bacterial growth was found in treatments at matched dosages of scrambled gapmers or plain oligonucleotide-free bolasomes compared to untreated cultures (5). Conclusion & Significance: Cationic bolasomes originally developed for the delivery of biologically active molecules to mammalian mitochondria (2-4) can successfully be used to deliver ASOs into bacteria. We also report the first successful in vitro antisense treatment to inhibit growth of C. difficile. The efficient delivery of antisense molecules via DQAsome-like nanovesicles into bacteria will allow the advantageous targeting of virulence functions essential for infection, disease, and recurrence.
Keynote Forum
Jyh-Ping Chen
Chang Gung University, Taiwan
Keynote: Controlled release of tissue plasminogen activator from magnetic nanocarriers in targeted thrombolysis
Time : TBA

Biography:
Jyh-Ping Chen has been a professor in Chemical and Materials Engineering at Chang Gung University since 1997. He holds joint appointments as a researcher in Department of Plastic and Reconstructive Surgery, Chang Gung Memorial Hospital. He received his PhD in Chemical Engineering from Pennsylvania State University in 1988. He joined Chang Gung University in 1994 and established the Graduate Institute of Biochemical and Biomedical Engineering in 2002 and served as the first director of the institute till 2006. He was the head of the Chemical and Materials Engineering Department from 2006 to 2014. Professor Chen has published more than 140 papers in SCI journals with more than 2900 citations. He is a guest editor or editorial board member for 12 international journals and a peer reviewer for more than 40 reputed journals. His current research interests include biomaterial, tissue engineering and drug delivery.
Abstract:
Thrombolytic drugs play a critical role in the treatment of various cardiovascular diseases including acute myocardial infarction, pulmonary embolism, deep vein thrombosis, arterial thrombosis and peripheral vascular thromboembolism. However, thrombolytic agents, such as tissue plasminogen activator, tend to dissolve both pathological thrombi and fibrin deposit at sites of vascular injury, resulting in hemorrhagic toxicity at therapeutic doses. Plasminogen activators also have short half-life and are immunogenic because of their foreign nature. By encapsulating proteins within novel carrier systems, an increased half-life and decreased immunogenicity might be obtained. Further, targeted delivery of thrombolytic agents followed by controlled drug release may reduce the risks of haemorrhage and toxicity associated with systemic administration, thus offering a promising, minimally invasive approach that could control and treat thrombosis. We have designed two magnetic nanocarrier systems for controlled release of recombinant tissue plasminogen activator (rtPA) in targeted thrombolysis. Ionic cross-linking of water-soluble chitosan with sodium tripolyphosphate in the presence of rtPA and magnetic nanoparticles could produce rtPA-encapsulated magnetic chitosan nanoparticles. Effective thrombolysis was demonstrated at an rtPA dose equivalent to 20% of the regular dose when the nanodrug was magnet-guided to the blood clot, followed by a triggered release of rtPA when switched to mobile magnetic guidance. An efficient thrombolytic drug delivery system using PEGylated thermosensitive magnetic liposome will be also discussed for magnetic targeted delivery of rtPA to the site of thrombus followed by temperature-triggered controlled drug release. The prepared nanodrug will be useful for magnetically guided target thrombolysis, followed by an alternating magnetic field for controlled release of rtPA by hyperthermic effects for treating thrombosis in a clinical setting.
Keynote Forum
Rossitza Lazova
California Skin Institute, USA
Keynote: Application of mass spectrometry imaging in the diagnosis of difficult melanocytic lesions
Time : TBA

Biography:
Rossitza Lazova trained in Dermatopathology with Dr. A. Bernard Ackerman. She was an Associate-Professor of Dermatology and Pathology and Director of the Dermatopathology Fellowship at the Department of Dermatology at Yale University for 20 years. Currently she is a Director of Dermatopathology at the California Skin Institute in San Jose, USA. Her main interest are melanocytic lesions and particularly Spitzoid neoplasms and melanoma. Dr. Lazova founded the Yale Spitzoid Neoplasm Repository and introduced Mass Spectrometry Imaging as an ancillary method in the diagnosis of difficult melanocytic lesions. Dr. Lazova has directed numerous sessions at national and international meetings in the US and throughout the world and has given hundreds of presentations worldwide. She has over one hundred publications including two textbooks and numerous chapters. She is on the editorial board of many reputable scientific journals. Dr. Lazova served as Vice President of the International Society of Dermatopathology.
Abstract:
In a previous study we identified differences on a proteomic level between Spitz nevus (SN), a type of benign mole, and Spitzoid melanoma (SM), melanoma that histologically mimics SN. Five peptides, comprising a specific proteomic signature, were differentially expressed by the melanocytic component of SN and SM in formalin-fixed, paraffin-embedded tissue samples. We sought to determine whether mass spectrometry imaging (MSI) could assist in the diagnosis and risk stratification of Atypical Spitzoid Neoplasms (ASN), lesions that show histologic features of both, benign SN and SM, and for which a definitive diagnosis of benign or malignant cannot be made with absolute certainty.
We performed MSI in a large series of cases of ASNs with known clinical follow-up. In each case we compared the diagnosis rendered by MSI with the histopathologic diagnosis and also correlated the diagnoses with clinical outcome. Patients were divided into 4 clinical groups representing best to worst clinical behavior. The association among MSI findings, histopathologic diagnoses, and clinical groups was assessed. When analyzing ASNs, for which neither melanoma nor nevus was favored histopathologically, MSI appeared to be more accurate in predicting the benign character of ASNs than histopathology and correlated better with their clinical behavior. Histopathology often overdiagnosed either atypical features or malignancy. We found a strong association between the diagnosis of SM by MSI and an adverse clinical outcome when clinical group 1 (no recurrence or metastasis beyond a sentinel node) was compared with groups 2, 3, and 4 (recurrence of disease, metastases or death). In addition, the diagnosis of SM by MSI was statistically strongly associated with adverse clinical behavior. MSI analysis using a proteomic signature may be able to provide more reliable diagnosis and clinically useful and statistically significant risk assessment of ASNs, beyond the information provided by histology and other ancillary techniques.
- Nanomedicine | Drug Delivery Research | Novel Drug Delivery Systems
Location: Hyatt Regency Osaka
Session Introduction
Jamboor Vishwanatha
UNT Health Science Center, USA
Title: Bone Microenvironment Targeted Nanoparticles for Metastatic Prostate Cancer Treatment

Biography:
Vishwanatha is a Regents Professor and Vice President for Diversity and International Programs, and Director of the Texas Center for Health Disparities at the University of North Texas Health Science Center at Fort Worth. He is a principal investigator of the National Research Mentoring Network, a NIH Common Fund initiative to provide mentorship, networking and professional development for a diversified biomedical and behavioral workforce. Vishwanatha received his Ph.D. in biological sciences from the University of South Carolina in 1983.Vishwanatha’s research is in cancer molecular biology, experimental therapeutics and nanotechnology. His laboratory is investigating genetic markers that predict development of aggressive prostate and breast cancers, and nanotechnology-based therapies for breast and prostate cancers. Vishwanatha is actively involved in mentorship and networking programs to diversify the biomedical research workforce, and has mentored numerous undergraduate and graduate students from under represented groups in biomedical sciences. As the director of the Texas Center for Minority Health, Education, Research and Outreach (Texas Center for Health Disparities), a Center of Excellence funded by the National institutes of Health, he has directed health disparity research, education and community outreach programs. For the past 11 years, he has organized the annual Texas Conference on Health Disparities that attract national speakers and participants. He serves on the external advisory committees for University of Puerto Rico-Cayey, PR; St. Mary’s University, San Antonio, Texas; Alabama State University, Montgomery, Alabama; and Savannah State University, Savannah, Georgia.
Abstract:
Purpose The most common site of metastatic prostate cancer is the bone. These metastatic lesions are difficult to treat and often result in off target cytotoxicity from current chemotherapeutics. We hypothesize that targeted nanoparticles (NPs) designed to deliver chemotherapeutics to cancer lesions in the bone microenvironment could improve treatment and the side effect profile that results from non-discriminate action of cytotoxic agents.
We have designed a novel targeted nanotherapeutic system to target the bone microenvironment in an effort to more efficiently deliver chemotherapeutics to the site of metastasis. The core of the NPs are composed of poly (D,L-lactic-co-glycolic acid) (PLGA) biodegradable polymer. The PLGA NPs have been loaded with the microtubule inhibitor, cabazitaxel. The surface of the NP has been conjugated with an amino-bisphosphonate through a BS3 (bis(sulfosuccinimidyl) suberate) linker system, which allows for high affinity binding to the hydroxyapatite structure of the bone.
Materials & Methods: NPs were formulated using a modified water-in oil-in-water double emulsion solvent evaporation technique. The physiochemical properties of the NPs were characterized. Ex vivo bone binding studies were performed. Cytotoxicity was tested in C4-2B and PC3 cell lines as well as in 3D tumor spheroids. Finally, NPs were tested for efficacy in an intraosseous tumor model of metastatic prostate cancer in athymic nude male mice.
Results: NPs were made with favorable physiochemical characteristics: mean hydrodynamic diameter of 236.8 nm ± S.D. 1.19 and mean polydispersity of 0.121 ± SEM 0.003. Cellular cytoxicity assay showed that C4-2B cells were more sensitive to the free cabazitaxel, the non-targeted NPs, and the targeted NPs compared to PC-3 cells. We did not see an appreciable difference between the targeted NPs and equivalent treatment of free cabazitaxel in 3D assays. In vivo analysis showed that both the non-targeted and targeted NPs were more effective than free cabazitaxel at reducing tumor burden. Additionally, targeted-NPs improved bone morphology at tumor lesions and were superior in behavioral tests.
Conclusions: In this project we have engineered a bone targeted NP formulation for metastatic prostate cancer. We have determined the chemical and physical characteristics of this system and tested the in vitro cytotoxicity. Finally, we have shown the efficacy of these targeted NPs in an intraosseous model of bone metastatic prostate cancer.
Acknowledgement : Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R21CA194295.
Michal Pechar
Czech Academy of Sciences, Czech Republic
Title: Polymer Cancerostatics With A Coiled Coil Motif Targeted Against Murine Leukemia

Biography:
Michal Pechar, PhD is a senior researcher in the Department of Biomedical Polymers, Institute of Macromolecular Chemistry of the Czech Academy of Sciences. He is the head of a research group investigating both actively and passively targeted macromolecular therapeutics and diagnostics. His main research interests involve polymer and peptide synthesis, design and preparation of new polymer drug delivery systems based on copolymers of N-(2-hydroxypropyl)methacrylamide and poly(ethylene glycol) and targeting with synthetic peptides or recombinant proteins. He is a co-author of 50 research articles in impacted journals and one patent with more than 1000 citations and H-index 19.
Abstract:
Coiled coil is a common structural motif in many natural proteins. It can be also utilized in design and preparation of the drug delivery systems for non-covalent connection of two macromolecules. In this work, two different pairs of peptides forming coiled coil heterodimers were designed, synthesized and characterized. While the peptide sequences (VAALEKE)4 (peptide EKE) and (VAALKEK)4 (peptide KEK) form a coiled coil heterodimer with random orientation of the peptide chains,(IAALESE)2-IAALESKIAALESE(peptideESE) and IAALKSKIAALKSE-(IAALKSK)2 (peptide KSK) tend to adopt an anti-parallel orientation of the chains. The orientation of the peptide chains in the coiled coil heterodimers was determined using fluorescence spectroscopy with fluorescence resonance energy transfer labels attached to the ends of the peptides. Both coiled coil heterodimers were used for attachment of a recombinant targeting protein – a single-chain antibody fragment of B1 antibody – to a polymer drug conjugate based on N-(2-hydroxypropyl)-methacrylamide bearing an anti-cancer drug pirarubicin (THP).
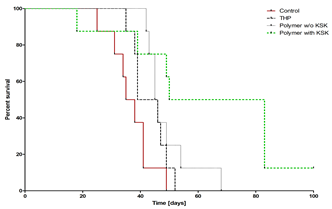
Figure 1. Survival of mice with BCL1 leukemia treated with free THP, non-targeted polymer-THP conjugate and targeted polymer-THP conjugate.
Both targeted polymer conjugates exhibited a markedly increased cytotoxic activity in vitro against BCL1 leukemia cells expressing the corresponding antigen compared to the non-targeted polymer drug conjugate. The targeted conjugate containing KSK/ESE coiled coil heterodimer (with the anti-parallel orientation) showed about 2-times higher cytotoxic activity and approximately 4-times higher cell-binding activity (as determined by flow cytometry) than the targeted conjugate with KEK/EKE anchor. In vivo therapeutic activity of the actively targeted polymer-THP conjugate (with KSK/ESE heterodimer) in mice bearing BCL1 leukemia was significantly higher (in terms of the survival time) compared with both the non-targeted polymer-pirarubicin conjugate and the parent drug (Figure 1). It was clearly demonstrated that the coiled coil heterodimers can be utilized for non-covalent attachment of recombinant targeting proteins to polymer-drug conjugates thus enabling preparation of a new generation of actively targeted macromolecular cancerostatics.
Galia Blum
The Hebrew University of Jerusalem, Israel
Title: Cathepsin Nanofibers Substrates for Targeted Drug Delivery

Biography:
Professor Blum’s main research focus is on the generation of novel chemical probes targeted to proteolytic enzymes and their application for medical uses such as molecular imaging and therapy. She also applies her original probes for basic research investigating the involvement of proteases in normal and pathological conditions. Over the years at the Hebrew University her group among other projects has developed novel probes for caspase-3 and discovered its activity in the ER, (Shaulove-Rotem 2016); and developed novel photodynamic probes targeted to cathepsin proteases and used them for combined detection and therapy of cancer (Ben-Nun 2015). Recently, she began also work on the nano-scale materials where she developed novel protease sensitive drug delivery molecules (Ben-Nun 2016) and nano-probes for CT molecular imaging.
Abstract:
The development of reactive drug carriers that could actively respond to biological signals is a challenging task. Different peptides can self-assemble into biocompatible nanostructures of various functionalities, including drugs carriers. Minimal building blocks, such as diphenylalanine, readily form ordered nanostructures. Here we present development of self-assembled tetra-peptides that include the diphenylalanine motif, serving as substrates of the cathepsin proteases. This is of great clinical importance as cathepsins, whose activity and expression are highly elevated in cancer and other pathologies, have been shown to serve as efficient enzymes for therapeutic release. Based on the cathepsins affinity around the active site, we generated a library of Phe-Phe-Lys-Phe (FFKF) tetra-peptide substrates (TPSs). We inserted various N-termini capping groups with different chemical properties to investigate the effect on protease affinity and self-assembly. All nine TPSs were cleaved by their targets, cathepsins B and L. However, solvent switching led to nanofibers self-assembly of only seven of them. Due to its rapid self-assembly and complete degradation by cathepsin B, we focused on TPS4, Cbz-FFKF-OH. Degradation of TPS4 nanofibers by cathepsin B led to the release of 91.8±0.3% of the incorporated anti-cancerous drug Doxorubicin from the nanofibers within eight hours while only 55±0.2% was released without enzyme treatment.
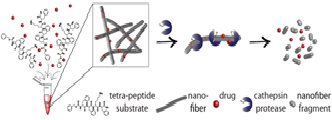
Finally, we demonstrated that tumor lysates fully degraded TPS4 nanofibers. Collectively, these results suggest that tetra-peptide substrates that form nanostructures could serve as a promising platform for targeted drug delivery to pathologies in which protease activity is highly elevated.
Rene J P Musters
VU University Medical Center, The Netherlands
Title: Ultrasound microbubbles: unique vehicles for targeted delivery of therapeutic molecules

Biography:
Rene Musters received a Master degree in Molecular Cell Biology & Electron Microscopy at the Utrecht University in 1990, where he also completed his PhD in 1994 (Thesis: "Ischemia and Phospholipid Reorganization in the Sarcolemma"). After his first post-doc position at the Department of Physiology at the ICaR-VU in Amsterdam (1994-1998) he specialized further in setting-up translational research in the field of cardiac adaptation at the Cardiovascular Research Laboratory (Department of Surgery) at the University of Colorado Health Sciences Center (UCHSC) in Denver (CO, USA). He returned to Amsterdam in 2000 where he was appointed Assistant Professor at the Department of Physiology of the VU University Medical Center in 2001. In 2002 he set-up the ICaR-VU 3D live-cell imaging facility at the Department of Physiology and continued to set-up multiple lines of translational research in collaboration with several clinical and pre-clinical departments and research groups. In 2016 he became Head of the Advanced Microscopy core facility in O|2 (AO|2M) at VU University Medical Center (see also www.ao2m.amsterdam ).
Abstract:
The development of ultrasound contrast agents containing encapsulated microbubbles has increased the possibilities not only for diagnostic imaging, but also for therapeutic applications. Microbubbles have been shown to be able to carry drugs and genes, and destruction of the microbubbles by targeted ultrasound results in local release of their therapeutic contents. Furthermore, ligands as well as nanoparticles can be attached to microbubbles so that they can be targeted to a specific target tissue or even target cells. In this presentation, recent advances of ultrasound microbubbles as vehicles for delivery of therapeutic molecules will be highlighted.
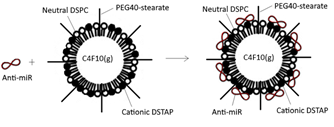
After adding antagomiR to positively charged microbubbles, microbubble-antagomiR complexes are formed.
HL-1 cells treated with ultrasound microbubbles.

Biography:
Iza Radecka joined the School of Sciences at the University of Wolverhampton, UK in 2000. Her research is focused on the cost-effective synthesis of new biomaterials using bacterial biopolymers, produced from bacteria using eco-sustainable feedstock and their chemical derivatization which can transform the crude polymer into a range of highly valuable products. She has published numerous research papers in highly ranked scientific journals, authored several chapters in biotechnological books. Iza has also given a broad number of invited lectures at international conferences. She has coordinated and participated to funded research projects at the EU as well as national level in the area of biopolymers and bioactive cements. Iza teaches on a wide variety of microbiology and biotechnology courses, both undegraduate and postgraduate level where she puts her knowledge and experience to good effect.
Abstract:
Statement of the Problem: In recent years, there has been an increasing interest in adenoviral vector anti-cancer therapy. The induction of an immune response, high liver deposition and lack of tumor tropism upon systemic delivery, can be considered as major limitations with this kind of application. Moreover, the ability of beneficial viruses such as bacteriophages to persist for extended periods is limited by many factors including: sunlight, irradiation and temperature. Bacteriophages are viruses that invade specific bacteria and kill them. Their high specificity and their safety profile make them particularly attractive natural antibacterial products. However, significant research efforts have been focused on development of poly gamma glutamic acid (γ-PGA)-based micro/nanoparticles used as a vector. The biopolymer γ-PGA is an extracellular bacterial polymer, it is biodegradable, non-toxic and non-immunogenic polymer. In this study, γ-PGA was used to protect bacteriophage from harmful environmental conditions. Also, we introduced an antibody blind polymer coated-viral vector. Methodology & Theoretical Orientation: Bacterial synthesis of γ-PGA was performed in a fermenter. Produced polymer was identified by Fourier Transforming Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR). The number average molecular mass (Mn) was determined by conventional aqueous based gel permeation chromatography (GPC). Different methods were performed to evaluate the possibility of using these micro/nanoparticles as a viral vector. These include single-emulsion method, simple ionic-gelation method and self-assembled NPs using precipitation and dialysis method.
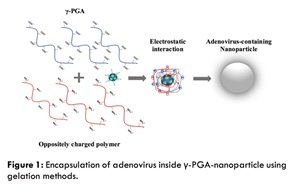
Findings: Bacteriophage formulated with 1% γ-PGA showed significant increase in survival rate compared to non-formulated phage after the exposure to extreme environmental conditions, such as heat, different pH and UV light, over a period of time. Adenovirus was successfully encapsulated inside the biopolymer with encapsulation efficiency of 92% with particle size of 565 nm. The cytotoxicity study showed that the particles are not toxic. The results obtained and the unique characteristics of the polymer established in this research could provide reference for coating and controlled releasing of viral vector used in anti-cancer therapy.
Aleksander F. Sikorski
University of Wrocław, Poland
Title: Liposomes targeted with therapeutic antibodies: a potent tool in anticancer therapy

Biography:
Aleksander F. Sikorski is a professor in biochemistry and cell biology at the Faculty of Biotechnology, University of Wrocław. His major scientific interests is membrane biochemistry, in particular, structure and function and biological role of spectrins as well as in lateral organization of biological membranes. His long lasting interest is in application of liposomes as drug carriers. He has been an author and co-author of more than 130 scientific papers (great majority published in peer-reviewed journals) and supervised 24 Ph. D. students who successfully obtained their degrees.
He is one of founding editors and since 2016 Editor-in-Chief of the international journal “Cellular and Molecular Biology Letters” (Established in 1996, i.f. 1.7) which is now published in collaboration with BMC/ Springer Nature. He has been a team leader for about 25 years and a chair of the Department of Cytobiochemistry since 2001. He was a founding Head of Academic center for Biotechnology of Lipid Aggregates for 8 years (2002-2010).
Abstract:
Statement of the Problem: Nanoparticle-based drug formulations are expected to be more efficient and less toxic than conventional drug formulations. This is indeed true in case of the most widely used nanoparticles including liposomes, micelles, dendrimers, nanotubes, and polymers. Moreover, most of the nanoparticle-based drug formulations offer a possibility of targeting the drug-loaded vehicles. Such strategy in case of anticancer drugs promises to be a hopeful strategy that allows to reduce toxicity and minimize adverse side effects. Targeting exploits the high affinity of cell-surface-targeted ligands, for specific retention and uptake by the targeted diseased cells.
Methodology & Theoretical Orientation: In this short review we would like to point to the application of liposomes as a versatile anticancer therapeutic carrier which can be targeted with antibodies directed against specific surface markers of cancer cells. Long-circulating liposomes containing PEG-PE and chemically activated PEG (e.g. maleimide derivative) may be considered as “Lego blocks”. Combining them with other components (i.e. drugs and surface-exposed molecules) in different configurations opens up multiple possibilities for different formulations of targeted anticancer drugs. They can be directed to specific tumor cells via targeting ligand(s) which could be attached covalently to the surface of liposomes. Prominent, relatively easy to apply as targeting agents are therapeutic antibodies already available on the market. Such liposomes may contain actively or passively encapsulated therapeutics of various nature.
Conclusion & Significance: Two types of such nanocarriers were developed in our laboratory. One consists of BCL-2 antisense oligodeoxynucleotide complexed with cationic lipid or polyethyleneimine with anti CD20 antibody and the other is based on simvastatin carrying liposomes targeted with anti HER2 antibody. The former type of formulations fulfill criteria of size, stability, specificity and high efficacy against specific type of cancer cells without obvious side effects in both in vitro and in vivo studies. Liposomal formulation of simvastatin targeted with HER2 antibody proves promising vehicle to deliver relatively high amounts of simvastatin to HER2 overexpressing cells.
Acknowledgement
The work was supported by grants from: Wroclaw Research Centre EIT+, project:“ Biotechnologies and advanced medical technologies” - BioMed (POIG.01.01.02-02-003/08) co-financed by the EU Operational Programme Innovative Economy,1.1.2) and by National Science Centre, Poland, grant UMO-2016/21/B/NZ7/01070 and by Wroclaw Center of Biotechnology, program The Leading National Research Center (KNOW) for years 2014–2018.
David Passlick
Max Planck Institute for Polymer Research, Germany
Title: Adjuvant combinations in protein-based nanocapsules induce superadditive stimulation of dendritic cells, and highly effective T cells responses

Biography:
David Passlick studied Molecular Biology at the Westphalian University of Applied Science (B.Sc.) in Recklinghausen and Biomedicine at the Johannes Gutenberg-University (M.Sc.) in Mainz. He is a doctoral candidate at the Max Planck Institute for Polymer Research and the University Medical Center in Mainz and is funded by the Max Planck Graduate Center. His interdisciplinary PhD project is focused on the enhancement of vaccination by nanocarriers for immunotherapeutic intervention in cancer and infectious diseases.
Abstract:
One aspect of vaccine development is to combine distinctly acting adjuvants to archive superadditive effects on immune cell activation [1, 2]. Moreover, a functional vaccine also requires an antigen source to enable the induction of T cell responses via antigen-presenting cells, particularly dendritic cells (DC), which are capable to activate even naïve T cells.

Figure
Schematic illustration of the project approaches (1) Identification of MDP+R848 as a suitable, superadditive adjuvant combination for BMDC. Soluble as well as particulated delivery induces upregulation of surface activation markers CD80/CD86. (2) Adjuvant delivery via OVA-nanocapsules mediates also strong antigen-specific activation of CD4+ and CD8+ T cells via MHC.
A promising approach to meet this challenge is the application of nanoparticles as a drug-delivery-system. In a first initial step, we analyzed and compared the immunostimulatory potential of different soluble TLR and NLR ligand combinations. We identified resiquimod (R848, specific for TLR7) [3] and muramyldipeptide (MDP, specific for NOD2) [4] as a superadditive stimulatory adjuvant combination. Particulated in spermine-modified dextran-nanoparticles, the combination R848+MDP stimulate murine bone marrow-derived dendritic cells (BMDC) stronger than the soluble adjuvant application. The second step was to combine adjuvants and antigen in one nanocarrier. For this purpose, we encapsulated the experimentally evaluated adjuvant combination (R848+MDP) in well-characterized [5] polymeric nanocapsules, whose shell consists of cross-linked ovalbumin (OVA) protein. OVA is a commonly used model antigen. To assess the capsules’ immunostimulatory potential, we treated BMDC with the adjuvant-loaded nanoparticles. The expression of the surface activation markers CD80/CD86 and the secretion of proinflammatory cytokines were measured by flow cytometry and cytometric bead array, respectively. The capability of the adjuvant-loaded OVA-nanocapsules to mediate OVA-specific T cell responses was assessed by performing T cell proliferation assays with transgenic OT-I (CD8+) and OT-II (CD4+) T cells that recognize OVA-derived antigens. Our data showed that particulated co-delivery of R848 and MDP activates murine BMDC in a superadditive manner as compared with single-delivery of the adjuvants. Additionally, the application of R848+MDP via OVA-nanocapsules evoked a strong antigen-specific T cell proliferation. These results show that R848/MDP-loaded OVA-nanocapsules are highly active to induce superior antigen-specific T cell responses.
Chi Hin Cho
Chinese University of Hong Kong, Hong Kong, China
Title: Vascular-targeted TNFα and IFNγ inhibited orthotopic colorectal tumor growth

Biography:
Chi Hin Cho is a gastrointestinal (GI) pharmacologist. Currently, he is the Research and Emeritus Professor in the Chinese University of Hong Kong, Hong Kong, China. He has the passion to unveil the various environmental risk factors including alcohol drinking, cigarette smoking and Helicobacter pylori infection in the pathogenesis of different disorders in the GI tract, in order to define the different effective therapeutic options in the treatment of GI diseases. Lately he moves toward more translational research, in particular using small peptides as probes linking up with different anti-cancer agents, targeting tumor vasculature and macrophages for stomach and colon cancer therapies. His working experiences in the hospital and universities as an administrator and teacher and also his research accomplishments in inflammation and cancer in the GI tract have made him one of the icons in the field for over 40 years
Abstract:
Tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) were originally identified to show potent anti-tumor activity and immunomodulatory capability. Unfortunately, several clinical studies of relevant cancer therapy did not observe significant response in maximum tolerated dose whether given alone or in combination. This unfavorable outcome was largely due to the nonspecific action and widespread systemic toxicity found in the body. We used a phage display technology to identify a tumor vasculature homing peptide (TCP-1) which targeted mainly at the vasculature of colorectal tumors but not normal blood vessels in animals and humans. With this discovery, we biologically conjugated the two immunomodulators individually with TCP-1 in order to provide a targeted therapy and perhaps also lessen the systemic side effects incurred by TNFα and IFNγ when given alone or combined treatment. In this study, we determined the antitumor effect and systemic toxicity of the new conjugates TCP-1/TNFα and TCP-1/IFNγ either given alone or in combination in an orthotopic colorectal tumor model in mice.

Targeted delivery of TNFα or IFNγ by TCP-1 peptide exhibited better antitumor activity than unconjugated moieties by inducing more tumor apoptosis and also enhancing antitumor immunity shown by increased infiltration of T lymphocytes inside the tumor. TCP-1/TNFα also normalized tumor blood vessels and increased anti-cancer drug concentration in the tumor (Figure 1). Interestingly combined therapy with TCP-1/TNFα and TCP-1/IFNγ synergistically suppressed tumor growth and alleviated systemic toxicities associated with untargeted therapy. This combination of drug treatment induced massive apoptosis/secondary necrosis in tumors. Taken together, our data demonstrates TCP-1 is an efficient drug carrier for targeted therapy of colorectal cancer (CRC). TCP-1/TNFα combined with TCP-1/IFNγ is a promising combination of immunotherapy for CRC.
Shunsaku Kimura
Kyoto University, Japan
Title: Peptide-based nanoparticles of immune-evasive, immune-tolerable, and immune-active properties

Biography:
Shunsaku Kimura has his expertise in peptideengineering in providing various kinds of peptide materials. He has invented to prepare molecular assemblies showing various morphologies of planar sheet, vesicle, and tube with homogeneous size distributions. This method is now extended to prepare chimeric morphologies including a round-bottom-shaped morphology which is considered as a combined morphology of vesicle and tube. One of the molecular assembly has been successfully applied for the theranostics of solid tumors, where the nanoparticle is labeled with indium-111 for SPECT imaging and with yttrium-90 for therapy. Breast cancer, bone cancer, brain cancer, and meningeal seeding can be nicely targeted with this nanoparticle. This approach paves a challenging aspect of nanoparticle based on the new concept of molecular design taking immune response into consideration.
Abstract:
Statement of the Problem: There are several candidates for a carrying vehicle of imaging and therapeutic agents targeting various kinds of tumors in the field of the emerging theranositcs. One effective vehicle is fully human antibodies, however, which cost high to threaten the preservation of the universal health insurance in Japan. Nanoparticles is the other potential candidate for the vehicle owing to the passive accumulation in solid tumors so called the EPR effect. However, nanoparticles should resolve the serious problem of the accelerated blood clearance (ABC) phenomenon which is the immune response triggered by nanoparticles. The purpose of this study is to demonstrate the designs of nanoparticles displaying immune-evasive, immune-tolerable, and immune-active properties. Methodology: Nanoparticles were prepared by the method of molecular assembling. The component molecules were amphiphilicpolypeptides and polydepsipeptides having poly(sarcosine) as a hydrophilic block. Findings: Nanoparticles having a densely packed hydrophilic layer around the nanoparticles were shown to evade from the immune system. When the nanoparticles were intravenously injected in tumor-bearing mice, they were found to accumulate in the tumor region at the 1st and 2nd doses similarly.

On the other hand, another nanoparticle, which was recognized by the immune system, was converted to the immune-tolerable nanoparticle by decorating the surface with sialic acid derivatives. Further, the immune-active nanoparticle was examined with using Ley as an antigen of solid tumors. The nanosheet expressing Ley on the surface was shown to be the most effective to trigger the immune system.
Conclusion & Significance: Nanoparticles intrinsically triggers the immune system, but can escape by a suitable surface design. The nanoparticles can be immune-tolerable by incorporation of sialic acids. Further, the antigen-carrying nanoparticle can be applied for cancer vaccination. Taken together, nanoparticles are highly potential in cancer medicine, resulting in support for the universal health insurance.
Konstantin Bloch
Felsenstein Medical Research Center, Tel Aviv University, Israel
Title: Islet-based insulin delivery to the brain for treatment of cognitive and metabolic disorders

Biography:
Konstantin Bloch, PhD is head of research team at Felsenstein Medical Research Center and associate professor at the Sackler Faculty of Medicine, Tel-Aviv University, Israel. Dr. Bloch is an expert in the field of pancreatic islet transplantation and cell immunoisolation. He is the scientific co-founder of the Beta-O2 Technologies, a biomedical company developing a highly oxygenated implantable bio-artificial pancreas for treatment of type 1 diabetes (currently in Phase I/II). Recently, his team developed a proprietary technology of cell-based insulin delivery to the brain for treatment of cognitive disorders.
Abstract:
Background: There is increasing evidence supporting a link between dementia associated with peripheral metabolic dysfunctions and impaired brain insulin signaling. Insulin therapy has previously been tested as an approach to ameliorate brain insulin resistance and deficiency in patients with various brain disorders. However, current strategies for insulin delivery to the brain may induce severe hypoglycemia when injected peripherally or show poor uptake when delivered intranasally. Recently, we have shown that intracranial transplantation of a small amount of naked or alginate immunoisolated pancreatic islets increased brain insulin content and attenuated cognitive dysfunctions without altering peripheral glucose homeostasis in rats with schizophrenia–like syndrome. In this study, we used a small number of intracranially grafted pancreatic islets for efficient and metabolically regulated delivery of insulin to the brain for treatment of cognitive and peripheral dysfunctions in a rat model of sporadic Alzheimer's disease (AD).

Results: The effect of intracranially grafted islets on cognitive and metabolic dysfunctions was tested using inbred Lewis rats with AD induced by a single intracerebroventricular administration of 3 mg/kg streptozotocin (icv-STZ). Six weeks after icv-STZ, the obese rats were transplanted with one hundred islets in the cranial subarachnoid cavity. Eight weeks after islet transplantation, the spatial learning and memory of the recipients as estimated by the Morris Water Maze test were significantly improved compared to non-transplanted icv-STZ rats. In addition, the transplanted icv-STZ rats demonstrated statistically significant reduction of food consumption, body weight and blood level of insulin compared to non-transplanted icv-STZ rats. Importantly, intracranially grafted islets increased brain insulin content without alteration in peripheral glucose homeostasis.
In conclusion: Our results provide a novel approach for efficient and metabolically regulated insulin delivery to the brain. Intracranial transplantation of a small amount of pancreatic islets attenuates cognitive decline and obese-related peripheral metabolic dysfunctions in a rat model of sporadic AD.
Roberto De Santis
IPCB-CNR, Italy
Title: Rapid prototyped nanocomposite magnetic scaffolds for tissue regeneration

Biography:
Roberto De Santis was born in Naples, Italy, in 1966. He received the mechanical engineering degree in 1994, the PhD in Biotechnology of Dental Materials in 1997 and the Master in Biomaterials in 2001. He is Researcher at the Institute of Polymers, Composites and Biomaterials (IPCB) of the Italian National Research Council from 2001. He is adjunct professor of Science and Technology of Dental Materials at the School of Dentistry, Faculty of Medicine of Naples “Federico II”. He has worked for many years on materials for biomedical applications developing particular skills on the properties of natural tissues, design of prostheses for hard tissues replacements and scaffolds for tissue engineering.
Abstract:
Statement of the Problem: Magnetic feature have been recently incorporated into polymer based multifunctional scaffolds for tissue engineering. The rationale relies on the possibility to deliver, on demand, bioaggregates such as drugs and growth factors, by switching on and off an external magnetic field. Moreover, cell seeding into these scaffold may be improved through external magnetic fields. Nanocomposite magnetic scaffolds consist of a thermoplastic polymeric matrix reinforced and functionalized with magnetic nanoparticles (MNP). These composites show a superparamagnetic behavior, as they magnetize in the presence of a magnetic field in a similar fashion of ferromagnetic materials, but removing the external magnetic field the residual magnetization is almost null.Methodology & Theoretical Orientation: Iron oxide and iron doped hydroxyapatite MNPs have been incorporated into aliphatic polyester matrix, and these nanocomposites were processed according to rapid prototyping techniques. A multiphysical approach based on magnetic measurements, simulations, mechanical testing and contact angle measurements has been carried out for characterizing these fully interconnected scaffolds. Cell-material interaction has been evaluated in vitro through cell assays, while preliminary in vivo behavior has been assessed through animal models.

Findings: Nanocomposite magnetic scaffolds have been successfully processed through rapid prototyping techniques. These scaffolds show a superparamagnetic behavior. MNPs allow to tailor mechanical properties and to improve wettability. Compared to neat aliphatic polyester based scaffolds, an enhancement of cell-material interaction and of tissue regeneration is observed. It seems that this approach is the only one possible to release, on demand, bioaggregates through an external physical signal.
Conclusion & Significance: Nanocomposite superparamagnetic scaffolds provide very unique features. Used in combination with magnetically labeled cells and/or magnetic functionalized bioaggregates, these scaffolds allow to trigger biological events by using static or dynamic magnetic fields. Custom made superparamagnetic nanocomposite scaffolds have the potential to guide the regeneration process of damaged biological tissues.
Yoav D Livney
Israel Institute of Technology, Israel
Title: β-casein nanovehicles for oral delivery of chemotherapeutic Drug combinations overcoming P-glycoprotein-mediated multidrug resistance in human gastric cancer cells

Biography:
Yoav D. Livney [B.Sc. (Suma cum Laude, 1990) Food Engineering & Biotechnology, Technion Israel Institute of Technology; M.S. (1995) Food Engineering, UW Madison, Wisconsin, USA; PhD (2002) Food Engineering & Biotechnology Technion IIT; Post-Doc Food Science, University of Guelph, Canada] of the Biotechnology and Food Engineering department, Technion [2004- Lecturer; 2007- Asst. Professor, 2012- Assoc. Prof.] is an expert in physical chemistry of biopolymers, and nano-deliverysystems for nutraceuticals and drugs. Authored >50 publications, 8 patents, gave >35 invited talks at international conferences, and mentored 13 M.Sc. and 7 Ph.D. students. Editorial-Board Member in several reputed journals.
Abstract:
Multidrug resistance (MDR) is a primary obstacle to curative cancer therapy. We have previously demonstrated that β-casein (β-CN) micelles (β-CM) can serve as nanovehicles for oral delivery and target-activated release of hydrophobic drugs in the stomach. Herein we introduce a novel nanosystem based on β-CM, to orally deliver a synergistic combination of a chemotherapeutic drug (Paclitaxel, PTX) and a P-glycoprotein-specific transport inhibitor (Tariquidar TQD) individually encapsulated within β-CM, for overcoming MDR in gastric cancer. Light microscopy, dynamic light scattering and zeta potential analyses revealed solubilization of these drugs by β-CN, suppressing drug crystallization. Spectrophotometry demonstrated high loading capacity and good encapsulation efficiency, whereas spectrofluorometry revealed high affinity of these drugs to β-CN. In vitro cytotoxicity assays exhibited remarkable synergistic efficacy against human MDR gastric carcinoma cells with P-glycoprotein overexpression (4). Oral delivery of β-CN based nanovehicles carrying synergistic drug combinations to the stomach constitutes a novel efficacious therapeutic system that may overcome MDR in gastric cancer.

Cytotoxicity (in terms of IC50 values) of PTX and PTX+0.8 μM TQD, with and without -CN encapsulation following simulated gastric digestion, examined using EPG85-257P, parental cell line and EPG85-257RDB multidrug resistant subline. N.S. indicates a non-significant difference, **indicates P < 0.001 and ***indicates P < 0.0001 as determined by student’s t-test.
Khalid AbdelRahim
King Saud university, Saudi Arabia
Title: Extracellular biosynthesis of silver nanoparticles using Rhizopus stonililifer

Biography:
Khalid AbdelRahim interested in antibiotics resistance problems. He is trying to help in overcoming this problem by awareness towards antibiotics usage, new drug by using nanotechnology.
Abstract:
Synthesis of silver nanoparticles (AgNPs) has become a necessary field of applied science. Biological method for synthesis of AgNPs by Rizobus stonilifer aqueous mycellical extract was used. The AgNPs were identified by UV–visible spectrometry, X-ray diffraction (XRD), Transmission electron microscopy (TEM) and Fourier transform Infrared spectrometry (FT-IR). Presence of surface plasmon band around 420 nm indicates AgNPs formation . The characteristic of the of AgNPs within the face-centered cubic (fcc) structure are indicated by the peaks of the X-ray diffraction (XRD) pattern corresponding to (111), (200) and (220) planes. Spherical, mono-dispersed and stable AgNPs with diameter around 9.47 nm were prepared and affirmed by high- resolution transmission electron microscopy (HR-TEM). Fourier Transform Infrared (FTIR) shows peaks at 1426 and 1684 cm-1 that affirm presence of coat covering protein the AgNPs which is known as capping proteins. Parameters optimization showed smallest size of AgNPs ( 2.86 ± 0.3 nm ) was obtained with 10−2 M AgNO3 at 40 º C. Present study provides the proof that the molecules within aqueous mycellial extract of R. stonilifer facilitate synthesis of AgNPs and highlight on value-added from R. stonilifer for cost effective. Also, eco-frindely medical and nanotechnology-based industries could also be provided. Size of prepared AgNPs could be controlled by temperature and AgNO3 concentration. Further studies are required to study effect of more parameters on size and morphology of AgNPs as this will help in control of large scale production of biogenic AgNPs.
James Hitchcock
University of Leeds, UK
Title: Impermeable metal nano-capsules for drug delivery without side effects

Biography:
James Hitchcock is currently focusing on colloidal science, in particular metallic nanoparticle manufacture, liquid core/polymer shell microcapsules, catalysts and metal electroless deposition. James’s 2015 paper ‘Long-Term Retention of Small, Volatile Molecular Species within Metallic Microcapsules’ successfully demonstrated, for the first time, the long term micro-encapsulation of small volatile molecules which lead to 8 patents being successfully filled and a ‘Research Excellent Award’. James is currently working to utilise this technology as a drug delivery vehicle successfully winning 2017 Welcome Trust ISSF Award and going through to the second round of a ‘Cancer Research UK Multidisciplinary Project Award 2017’ grant application.
Abstract:
Cancer therapeutics have dramatic side effects on healthy tissues. A prominent research area focuses on encapsulating cytotoxic drugs for targeted delivery to cancer tissues and for reduction of off-tissue side-effects. However, significant challenges remain for encapsulated clinical drugs (e.g. liposomal doxorubicin):
1. Drug encapsulation remains very expensive.
2. Drug loading is low and the manufacturing process is inefficient
3. Once encapsulated drug leaching over time is often high (especially on dilution)
4. Typically release specifically within tumours is not achieved
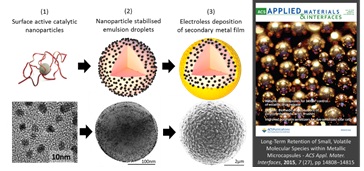
Schematic diagrams and corresponding electron microscopy images of the different phases: (1) catalytic metal nanoparticle synthesis, (2) nanoparticle stabilization of liquid emulsion droplets and (3) electroless deposition of a continuous, impermeable metal coating. An image of the metal capsules on the front cover of ACS Applied Materials and Interfaces is also included.
Recently, we have demonstrated the efficient manufacture of impermeable metal-shell/liquid core microcapsules (Hitchcock ‘Long-term Retention of Small, Volatile Molecular Species within Metallic Microcapsules’, ACS Appl. Mater. Interfaces, 2015, 7, 14808) that permit localised delivery of active (pharmaceutical) ingredient high doses by triggering release with ultrasound at the target location. This delivery method has the potential to be superior to all previously developed encapsulation strategies because it would address all of the above challenges simultaneously:
1. Capsules can easily be manufactured at industrial scale,
2. High drug content can be achieved within capsule cores,
3. No drug leaching occurs, thus preventing any side-effects prior to release activation via ultrasound treatment,
4. Complete release of high drug concentrations can be achieved in cancer-affected areas only.
Bruno Sarmento
University of Porto, Portugal
Title: Bioengineered nanomedicines for modulation of intestinal anti-diabetic peptide delivery

Biography:
Bruno Sarmento completed his PhD in Pharmaceutical Technology and degree in Pharmaceutical Sciences, University of Porto, Portugal; Affiliated Researcher at Institute of Investigation and Innovation in Health (i3S) and Institute of Biomedical Engineering (INEB), University of Porto, Portugal; Assistant Professor of Pharmaceutical and Biopharmaceutical Technology at IUCS, Gandra, Portugal. His current research is focused on the development of functionalized nanomedicines and their application in the pharmaceutical and biomedical fields. In particular, nanoformulations of biopharmaceutical drugs with interest in diabetes, cancer and infectious diseases. He has also specialized in mucosal tissue engineering models to validate functionalized nanomedicines and to perform in vitro/in vivo correlation. He published more than 160 papers in international peer reviewed (ISI) journals, most in top journals (Q1 in Pharmaceutical Sciences; Q1 in Nanoscience and Nanotechnology; total citations 3350; accumulated impact factor 627; H-index 29), 34 book chapters and more than 180 proceedings. He edited 4 books, participated in more than 50 invited/selected talks in national and international meetings and was awarded several distinctions. He is member of the Editorial Advisory Board of 10 international journals and has acted as referee for top-ranked journals in his area of expertise, and for international funding agencies.
Abstract:
Diabetes mellitus is a high prevalence and one of the most severe and lethal diseases in the world with tremendous impact on health worldwide. Anti-diabetic peptides as insulin or GLP-1 are commonly used to treat diabetes in order to give patients a better life condition. However, due to bioavailability problems, their most common route of administration is the subcutaneous route. Invasive delivery route is, still, the most efficient, but less desired by patients. Non-invasive delivery systems have potential to overcome the most pressing problem regarding effective treatment of diabetic patients - therapy compliance, and is thus considered as convenient alternative, but it faces important challenges. Therefore, the nanoencapsulation of antidiabetic peptides into nanoparticles is presented as a good strategy to improve bioavailability.
In our research group, we have developing and characterizing nanoparticles containing insulin and/or GLP-1 peptides, following their evaluation as medical products to control diabetes upon oral delivery. In particular, we use poly(lactic-co-glycolic acid) (PLGA) modified with chitosan and a cell-penetrating peptide. Afterwards, glucagon like peptide -1 (GLP-1) loaded NPs were encapsulated into a pH-sensitive polymer and loaded with dipeptidyl peptidase-4 (DPP4) inhibitor, using a microfluidics technique. The in vivo tests were performed in a rat type 2 diabetes mellitus model by oral gavage, and the blood glucose levels were quantified. The plasmatic insulin levels, as well as the pancreatic insulin content, were also evaluated.

Our no-invasive technologies have demonstrated a clear efficacy in lowering the blood glucose levels in diabetic animal models. Not only nanoparticles have demonstrated to be able to cross biological barriers, but also provide sustain release of their peptide payloads. The interaction between the nanoparticles and the intestinal co-culture cells showed that there was a clear increase in the interaction between the modified nanoparticles with the cells. In the in vivo assays, the blood glucose levels decreased after 4 h of the administration of the particles and were kept low thereafter. The insulin levels increased along the time and the insulin pancreatic content was higher in the experimental groups in comparison with the control. No inflammation, cytotoxicity or tissue damage have been associated with chronic use of such nanoparticles, giving promising clinical application in a near future.
The developed particles were sensitive to different pH, showing high interact with intestinal cells. They allowed the dual-delivery of two different drugs in a single formulation. The very low activity of DPP4 enzyme prolonged the GLP-1’s half-life, thus increasing the insulin levels and decreasing the blood glucose levels along the time in vivo.
In this presentation, it will be demonstrate the feasibility of nanomedicines to improve the bioavailability and efficacy of antidiabetic biopharmaceutical drugs.
Hidetoshi Arima
Kumamoto University, Japan
Title: Potential Use of Folate-appended Methyl-β-cyclodextrin as a Novel Antitumor Agent Inducing Mitophagy

Biography:
Hidetoshi Arima is Professor of Graduate School of Pharmaceutical Sciences, Kumamoto University, Japan. He received a Ph.D. in 1991 from Kumamoto University in Japan. From 1991 to 1993, he worked at Eisai Co., Ltd. in Japan. From 1993-1998, he worked at Tokyo University of Pharmacy and Life Sciences as research associate. In 1998, he moved to Faculty of Pharmaceutical Sciences in Kumamoto University in Japan, and then was promoted to associate professor in 2001. In 2007, he was promoted to Professor in Graduate School of Pharmaceutical Sciences, Kumamoto University in Japan. His research interest is design and evaluation of integrated drug delivery system (DDS) based on cyclodextrins. In addition, he started to research the potential use of sacran, a supergiant cyanobacterial polysaccharide, as natural drugs and DDS carriers. Over 150 research papers and 10 patents have been published since 1986. Further growth of the DDS research is expected.
Abstract:
Methyl-β-cyclodextrin (M-b-CyD) induced apoptosis in tumor cells and had the potential as a novel antitumor agent and/or its lead compound. To obtain a tumor cell-selectivity of M-β-CyD, we newly synthesized folate-appended M-β-CyD (FA-M-β-CyD), and evaluated the potential of FA-M-β-CyD as a novel antitumor agent in vitro and in vivo. In contrast to M-β-CyD, FA-M-β-CyD entered KB cells (folate receptor (FR)-α (+)) through CLIC/GEEC endocytosis in a FR-a-dependent manner , and provides selective antitumor activity in FR-a-expressing cells by the induction of autophagy, not apoptosis. FA-M-β-CyD drastically inhibited the tumor growth after intratumoral or intravenous injection to FR-positive Colon-26 cells-bearing mice. Importantly, an intravenous administration of FA-M-β-CyD to tumor-bearing mice did not show any significant change in blood chemistry values. To gain insight into the detailed mechanism of this antitumor activity,
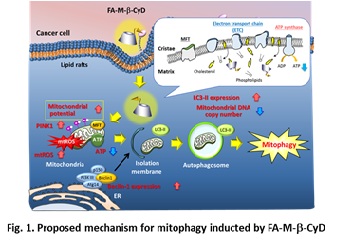
we focused on the induction of mitophagy by the treatment of FR-a-expressing tumor cells with FA-M-b-CyD. The transmembrane potential of isolated mitochondria after treatment with FA-M-b-CyD was significantly elevated. Additionally, FA-M-b-CyD lowered ATP production and promoted reactive oxygen species production in KB cells (FR-a (+)). Importantly, FA-M-b-CyD enhanced light chain 3 (LC3) conversion (LC3-I to LC3-II) in KB cells (FR-a (+)) and induced PINK1 protein expression, which is involved in the induction of mitophagy. Furthermore, FA-M-b-CyD had potent antitumor activity in BALB/c nu/nu mice xenografted with KB cells (FR-a (+)) without any significant side effects. Taken together, these findings demonstrate that the autophagic cell death elicited by FA-M-b-CyD could be associated with mitophagy induced by an impaired mitochondrial function, and has the potential as a novel antitumor agent inducing mitophagy.
Yareli Rojas-Aguirre
CCADET-UNAM, MEXICO
Title: The drug delivery (r)evolution: nanomedicine and 3D printing for personalized therapies

Biography:
Yareli Rojas-Aguirre got her PhD degree in Chemistry in 2013 at the National Autonomous University of Mexico. In the same year, she joined the Biomedical Engineering Department at the University of Michigan as a postdoctoral fellow. In 2014, she came back to Mexico to start her career as young professor at the National Autonomous University of Mexico. Her research interests focus on the design and synthesis of biomaterials and the study of their interactions at biological interfaces to: Rational design and develop advanced drug delivery systems. Investigate at the micro and macro scale biomaterials fabrication processes, in particular 3D printing (additive manufacturing) for biomedical devices. Integrate the biomaterials and biofabrication research with the discovery of new bioactive molecules and/or drug repositioning approaches to accomplish drug delivery platforms that lead up to novel and suitable therapies.
Abstract:
One of the main goals of nanomedicine is the design and development of “smart” drug delivery systems: nanostructures that can simultaneously diagnose and release bioactive molecules under spatio-temporal control. To that end, the multifunctional drug delivery platforms must display unique properties but also require sophisticated chemical approaches to build them. Although their fascinating molecular engineering has substantially enriched the literature, they could be quite far to reach the market. What is needed? Besides the construction of platforms with more functionalities, is fundamental to keep in mind key those aspects that can make the nanostructures succeed. On the other hand, the way the systems will be fabricated and scaled-up should not be neglected. One of the most outstanding fabrication techniques is 3D printing. The integration of nanomedicine and 3D printing has opened the door to a vast number of opportunities for manufacturing nanomaterials-based micro and macrosale biomedical devices.

In this context, the research is mainly focused on the design of novel inks comprised by smart materials, nanocomposites and even living cells. Certainly, innovative inks are needed, but is that enough? Thus, in this work, we present our approaches to answer the stated questions. Regarding the drug delivery platforms, our approach is the systematic design of nanosystems by studying every single gear of the nanoassembly in terms of their interactions at the biological interfaces, and the physicochemical evaluation of their disassembly when they reach the biological target. With respect to the 3D printing technologies, we are focused on the relationships between the manufacturing parameters/processes and the physicochemical requirements of the actual nanomaterials for being printed.
In this way, we intend to join two revolutionary directions in drug delivery, not for creating a super Revolution but to be part of the Evolution of the pharmaceutical and biomedical fields
Vincenzo Guarino
National Research Council of Italy, Italy
Title: Electrofluidinamics: a new toolbox to design instructive platforms for tissue engineering and molecular therapies

Biography:
Vincenzo Guarino, PhD in Biomaterials Science, is Senior Research Scientist at IPCB-CNR since 2006. He is expert of process technologies for scaffold design and electrofluidodynamic technologies (i.e, electrospinning, electrospraying, Electro Dynamic Atomization) to produce micro/nanofibres, particles and capsules for tissue regeneration, drug delivery and cancer therapy. He is author of over 70 international publications (H-Index 20 – Scopus source), 9 book chapters, 2 patents (1 pending) and about 200 communications in international/national conferences and awarded as author/co-author in several international congresses on biomaterials. He is also Editor/ Reviewer of several international journals, and Member of the European (ESB) and Italian (SIB) Society of Biomaterials and American Nano Society.
Abstract:
A large variety of processes and tools is recently emerging to design instructive materials with controlled chemical, physical and biological properties for tissue engineering and drug delivery. Among them, electro fluido dynamic techniques (EFDTs) are revolutionising traditional biomaterial’s manufacturing approach, basically identifing relatively complex fabrication processes to design innovative devices with restrained manufacturing costs but high functional complexity. By the use of electrostatic forces, biomaterials may be manipulated in different forms for the fabrication of a large set of 3D platforms with controlled micro/nanostructure (i.e., scaffolds, nanoparticles, capsules, mono or multicompartment systems, microgels and microscaffolds) able to more efficiently address in vitro and in vivo cell activities. EFDTs allow to produce fibres and/or particles at micro- and/or submicro-metric size scale through a rational selection of polymer solution properties and process conditions, thus creating different 3D devices able to incorporate biopolymers (i.e., proteins, polysaccharides) or active molecules (e.g., growth factors, chemotherapeutics) for countless applications in tissue engineering, drug delivery and cancer therapy. Here, we overview the basic EFDTs technologies – i.e., electrospinning, electrospraying and electrodynamic atomization – and recent current advances in experimental setups to develop bio-inspired platforms for tissue engineering, drug delivery and cancer therapy.
Miguel Moreno
Nanyang Technological University, Singapore
Title: Stability of ranibizumab and aflibercept for sustained release. Their delivery from thiolated chitosan-based hydrogels

Biography:
Miguel Moreno has a passionate interest in drug delivery and innovation management. He really looks forward to transferring his research to commercially feasible technology. He is a committed biomedical research scientist with over fifteen years’ experience in biophysics (protein-protein and protein-liposome interactions), biochemistry, polymer chemistry, drug delivery systems, sustained release, biomedical applications. He has developed a polymer-based platform for carrying peptides, bringing out a targeted pro-cytotoxic drug delivery system based on wasp venom for cancer therapy, which has been awarded. At present, he is involved in a large "ocular diseases" project, from NTU-Northwestern Institute for Nanomedicine (NNIN), for developing new carriers to deliver anti-inflamatory drugs and anti-VEGF proteins for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME). He has expertise in designing, optimizing and evaluating entrapment of therapeutic proteins, peptides and small molecules into a wide variety of nano- and microcarriers and hydrogels.
Abstract:
Statement of the problem: The anti-vascular endothelial growth factor (VEGF) agents such as ranibizumab (Lucentis®) and aflibercept (EyLea®) are currently used as monthly or bimonthly intravitreal injections to treat potentially blinding retinal diseases such as wet age-related macular degeneration (AMD) or diabetic macular edema (DME). Because of the complications associated with repeated intra-vitreal injections, there is considerable interest in developing a sustained delivery system. The purpose of this study was to examine the stability of both therapeutic proteins under physiological conditions as well as when incorporated into drug delivery systems (DDS). Furthermore, these two therapeutic proteins were entrapped into thiolated chitosan-based hydrogels and their release was modulated by different degree of crosslinking. A detailed study of characterization of released protein was performed.

Results: Using several complementary and stability-indicating analytical methods, we have demonstrated that both ranibizumab and aflibercept were stable in an extended range of different pH conditions as well aswhen incubated at 37°C for up to 30 days. In addition, we investigated the stability of both proteins after a rapid screening method that simulates the first homogenizing step during the protein microencapsulation process. Finally, optimal controlled release of active released proteins from thiolated chitosan-based hydrogels was characterized.
Conclusion & Significance: the stability of ranibizumab and afflibercept may be considered optimal for long-term sustained release, maintaining their capacity to bind VEGF. Controlled bioactive release for one month was achieved for both proteins in vitro using thiolated chitosan hydrogels. These ones may be an advantageous ocular carriers for improving wet AMD and DME.
Nianping Feng
Shanghai University of Traditional Chinese Medicine, China
Title: Chitosan-Modified Silymarin-Loaded Lipid-Polymer Hybrid Nanoparticles: Preparation and the Lipid-Lowering Effect

Biography:
Nianping Feng is currently a full Professor of Pharmaceutics at Shanghai University of Traditional Chinese Medicine (SHUTCM). He received his Ph. D from China Pharmaceutical University in June 1997 and joined the SHUTCM in October 1998. From September 2012 to September 2013, Dr. Feng worked as a Senior Research Scientist at Purdue University. His research interests include novel drug delivery system, nano pharmaceuticals, and TCM-based new drug development. Prof. Feng published more than 100 research papers and 8 book chapters and holds 10 patents.
Abstract:
Statement of the Problem: Nonalcoholic fatty liver disease (NAFLD) is one of the most commonly diagnosed chronic liver diseases. The identification of safe and effective drugs treating NAFLD is critical for improving patient survival and preventing further progression of the disease. Silymarin (SM) is a mixture of flavonoid compounds extracted from the seeds of Silybum marianum (L.) Gaertn, and has been used as an agent in the treatment of fatty liver, liver injury, and hepatitis, and has shown hepatoprotective and antioxidant properties. However, the poor water solubility of SM limits its oral absorption and bioavailability. The purpose of this study is to develop the chitosan-modified, SM-loaded LPNs (CS-LPNs) to enhance the bioavailability and improve the efficacy of SM.
Methodology: A NAFLD model was generated by using PNPLA3 I148M transgenic mice, and the lipid-lowering effect of chitosan-modified silymarin-loaded lipid-polymer hybrid nanoparticles was investigated. The nanoprecipitation method was used to prepare the lipid-polymer hybrid nanoparticles, and their characterization was also determined.
Findings: The average diameter of the CS-LPNs was 286.47 ± 23.79 nm. In vitro release experiments showed an 80% release within 8 h. Cytology, pharmacokinetics, and pharmacodynamics of the nanoparticles were characterized. CS-LPNs reduced lipid accumulation in the livers of PNPLA3 I148M transgenic mice, effectively lowered blood lipid levels, and improved liver function. CS-LPNs provide a new treatment option for NAFLD.
Conclusion: We confirmed that CS-LPNs can inhibit lipid accumulation in the liver and enhance drug efficacy. These findings indicate that CS-LPNs may offer a new treatment option for NAFLD.
Yaping Li
Chinese Academy of Sciences, China
Title: Intelligent Nanoparticles for Combination Photoimmunotherapy of Cancer

Biography:
Yaping Li received Ph.D. degrees in Fudan University in 2001. He devoted himself in drug targeted delivery based on nanotechnology, mainly involved in reversing MDR and improving pharmacological efficacy of antitumor agents, designing and constructing new non-viral vector for gene delivery. He has published over 150 scientific papers in Nat Medicine, Adv Mater, ACS Nano, Adv Funct Mater, Small, Biomaterials, Nanomedicine NBM, J Control Release, etc. He won National Science Fund for Distinguished Young Scholars (2009), Shanghai Outstanding Academic Leaders (2011), Zhu Li Yuehua Excellent Teacher Award of CAS (2010), the Hundred Talents Program Talent of CAS (2010) and Shanghai Leading Talent (2010). He is the Chief Scientist of National Basic Research Program of China in Nanoscience and Nanotechnology (2009), Vice Chairman of Pharmaceutics Society of Shanghai Pharmacy Association, Member of Council, Shanghai Pharmaceutical Association, Member of Branch of China in the International Controlled Release Association (CRS), and Member of the Chinese Pharmaceutical Associstion.
Abstract:
Photoimmunotherapy (PIT) has emerged as a promising clinical modality for cancer therapy due to its ability to initiate an antitumor immune response. However, PIT is severely impaired by tumor cell immunosuppression of host T-cell antitumor activity through the programmed cell death 1 ligand (PD-L1) and programmed cell death receptor 1 (PD-1) (PD-L1/PD-1) immune checkpoint pathway. In this study we demonstrate that PIT can be augmented by PD-L1 knockdown (KD) in tumor cells. We rationally designed a versatile micelleplex by integrating an acid-activatable cationic micelle, photosensitizer (PS), and small interfering RNA (siRNA).
The micelleplex was inert at physiological pH conditions and activated only upon internalization in the acidic endocytic vesicles of tumor cells for fluorescence imaging and PIT. The combination of PIT and PD-L1 KD showed significantly enhanced efficacy to inhibit tumor growth and distant metastasis in a B16-F10 melanoma xenograft tumor model. These results suggest that acid-activatable micelleplexes utilizing PDT-induced cancer immunotherapy are more effective when combined with siRNA-mediated PD-L1 blockade.
Young Tag Ko
Gachon University, South Korea
Title: Mitochondrial-Targeted Photosensitizer-Loaded Albumin Nanoparticle for Photodynamic Therapy of Malignant Brain Tumors

Biography:
Young Tag Ko has his research expertise with nanomedicines and drug delivery systems for nucleic acid therapeutics. Of my research interests are drug delivery systems, in particular, in vivo delivery of nucleic acid therapeutics (pDNA, ODN, RNA) using nanoparticular systems of lipids, polymers, and lipid-polymer combinatory systems such as artificial virus, lipid-modified polymers, and capable of tissue and disease-specific targeting, in particular brain and cardiovascular systems. Understanding the intracellular and extracelluar fate of such systems using various in vitro and in vivo imaging techniques is also of my interest.
Abstract:
Introduction: Photodynamic therapy (PDT) is a promising approach for the treatment of serious disorders including cancer. The objective of this study was to develop a mitochondria-targeted photosensitizer (PS) for PDT. The PDT efficiency of PSs can be enhanced by improving its intracellular targeting ability. The lifetime of singlet oxygen generated from PS by light treatment is between 10–320 nanoseconds in the target area; therefore, its diffusion is only limited to approximately 10 nm to 55 nm in cells. Thus, the PS must be localized as close as possible to cellular organelles, such as the nucleus, mitochondria, and lysosome.
Methods: Herein, a porphyrin-derivative PS, pheophorbide a (PheoA), was conjugated to carboxybutyl triphenylphosphonium (TPP) via a carbodiimide linkage to enhance mitochondrial targeting and TPP-PheoA conjugate was further loaded into folate-cholesteryl albumin (FA-chol-BSA) nanoparticles (NPs) to improve its biocompatibility. In vitro and in vivo studies have been done in B16F10 and HeLa cancer cell lines and tumor-bearing mice, respectively, in the presence of laser irradiation. Furthermore, we generated orthotopic GBM xenograft mice by intracranially implanting luciferase gene transduced U-87 MG human glioblastoma cells and evaluated antitumor capacity of the NP in the tumor bearing mice. Real-time biodistribution was determined by monitoring the fluorescence of PS, and the growth of GBM after treatment was also determined by monitoring the bioluminescence signal from D-luciferin-luciferase using in vivo imaging system (IVIS).
Results: TPP-PheoA and TPP-PheoA@FA-chol-BSA NPs were readily taken up by B16F10 and HeLa cells. Further in vitro studies exhibited that TPP-PheoA and its nanoparticle primarily accumulate in the mitochondria, greatly generate ROS, lead mitochondrial disruption and cell apoptosis, and have higher phototoxicity against cancer cells. In vivo bioimaging and in vivo antitumor studies indicated that TPP-PheoA@FA-chol-BSA NP greatly accumulated in the tumor area and significantly suppress the tumor growth as compared to PheoA@FA-chol-BSA NP in tumor-bearing mice. TPP-Pheo@FA-chol-BSA NP also greatly accumulated in and significantly suppressed the growth of the brain tumor compared to the free PS and PBS in the orthotopic GBM model mice.
Conclusion: Taken together, TPP-PheoA@FA-chol-BSA NP could be a promising mitochondria-targeted PS for image-guided PDT and has great potential as a clinically applicable therapeutics for photodynamic therapy of GBM.
Zifu Li
National Engineering Research Center for Nanomedicine, Huazhong University of Science and Technology, China
Title: Combined administration by nanomedicine: an effective treatment against insufficient chemotherapy-promoted tumor metastasis

Biography:
Zifu Li is currently a Professor in College of Life Science and Technology at Huazhong University of Science and Technology. Dr. Li obtained his PhD, majored in polymer physics, in 2012 from the Chinese University of Hong Kong. Afterwards, he worked in University of Alberta as a postdoc. During March 2015 and January 2016, Dr. Li worked as a research scientist in Georgia Tech. Now, Dr. Li is focusing on hydroxyethyl starch (HES) based smart nanomedicine, for cancer immunotherapy applications.
Abstract:
Statement of the Problem: Although nanoparticles could enhance the delivery efficiency of chemotherapeutics, the percentages accumulating at tumor site is still low, with a median of 0.7% for the last decade. Tumor tissue with insufficient chemotherapy might be the crime culprit of tumor metastasis. In this research, we adopted a combined-administration strategy by nanoparticle to prevent the insufficient chemotherapy-promoted tumor metastasis. DOX and TGF-β receptor inhibitor, LY2157299, were co-delivered by HES-PLA nanoparticle. With suppression of EMT process by blocking the TGF-β path, the distant metastasis were dramatically inhibited. Meanwhile, DOX exhibited better anti-tumor efficacy on the primary tumor.
Methodology & Theoretical Orientation: HES-PLA nanoparticles was obtained by a three-step preparation procedure and characterized by TEM, AFM and DLS. Anti-tumor efficacy in vitro was evaluated with 4T1 cell by MTT assay. Western blot was applied to evaluate the expression of EMT-related proteins. ELISA was used to quantify TGF-β. The metastatic capabilities of cell treated with different administrations were evaluated by transwell assay (in vitro) and zebrafish model (in vivo). Mice bearing subcutaneous 4T1 tumor were ultimately used to examine its efficacy of anti-primary tumor and anti-distant metastasis.
Findings: The transwell assay and zebrafish model reveal that low dose of DOX could enhance the metastasis ability of 4T1 cell. Importantly, the in vivo study demonstrate that co-deliver of DOX and LY2157299 by nanoparticle could simultaneously suppress the primary tumor (TIR, 80.7%) and distant metastasis (no nodule on the lung).
Conclusion & Significance: In vivo biological barriers always deteriorate the delivery efficacy of DOX, causing insufficient chemotherapy and therefore promoting metastasis. Combined administration of DOX and LY2157299 could significantly suppress the insufficient-chemotherapy-enhanced EMT process by blocking the TGF-β path, decreasing the distant metastasis and intensifying the anti-tumor efficacy of DOX.
Erwan Rauwel
Tallinn University of Technology, Estonia
Title: Nanomaterial synthesis and study for biomedicine and cancer treatment

Biography:
Erwan Rauwel PhD, habil. obtained his PhD from Caen University in Materials science. He continued with postdoctoral studies at Minatec, Grenoble, France and the University of Aveiro, Portugal. He has been a Senior Researcher at University of Oslo where he has developped a new method for the synthesis of ultrastable metal nanoparticles (patented). Since, 2013, he is Professor at Tallinn University of Technology, Tartu College where his team is investigating the properties of these metal nanoparticles for biomedical applications. He has more than 50 peer-reviewed publications, 2 book chapters and 4 patents. He is presently consulting editor for International Journal of Nanomedicine.
Abstract:
The past 2 decades have seen the development of new methods for the production of nanoparticles. The main interests in nanoparticles comes from their unique chemical and electronic properties due to their large surface-to-volume ratio and their high potential applications. The advances in synthesis methods have improved thereby giving monodisperse nanoparticles with a good size distribution and shape control during the synthesis. However, the methods of synthesis strongly affect their intrinsic properties and more particularly the surface properties. In particular, the presence of surfactants or organic species on their surface can shield their properties. However, surfactant-free nanoparticles are very sensitive to their environment and more particularly in the human body. In addition, metal nanoparticles like cobalt, copper and nickel produced using conventional methods have pyrophoric properties. So, the encapsulation of nanoparticles into polymer with an extremely low cytotoxicity is usually necessary for the development of biomedical applications.
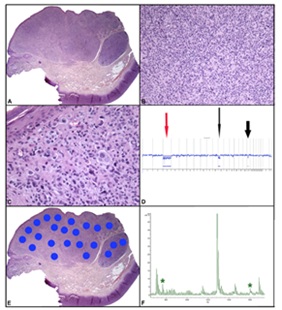
Biological approaches and green synthesis routes using biological molecules originating from plant extracts have been developed for the synthesis of nanoparticles thus, discarding the use of toxic chemicals. Here, the active molecules of plant extracts are used as active capping agents thereby improving the nanoparticle properties influenced by the plant extracts. The functionalization of the surface for targeted applications has sparked a lot of interest for cancer treatment and the development of biocidal coatings. This talk will highlight some of the advances and will emphasize on current nanomaterials that are presently developed for biomedical applications. Our recent results involving ultrastable metal nanoparticles will also be described.
Alejandro Sosnik
Israel Institute of Technology, ISRAEL
Title: Nanoparticle-in-Microparticle Delivery Systems for Oral Delivery of poorly water-soluble drugs: From concept to practice

Biography:
Alejandro Sosnik is Associate Professor of the Department of Materials Science and Engineering of Technion-Israel Institute of Technology since early 2014 where he founded the Laboratory of Pharmaceutical Nanomaterials Science. His current research lines comprise drug self-assembly and crystallization phenomena and processing, polymer and macromolecular chemistry, biomaterials science, colloidal chemistry (drug and polymer self-assembly), mucoadhesive drug delivery systems, nanomedicine (drug encapsulation, release and targeting), therapy of poverty-related diseases (HIV, tuberculosis), pediatric cancer, intestinal diseases and pharmacokinetics (oral, inhalatory and intranasal administration routes) in both preclinical and clinical trials.
Abstract:
Nanoparticle-in-Microparticle Delivery Systems (NiMDSs) are a novel kind of delivery platform composed of a drug that is encapsulated within a nanoparticle and is then, re-encapsulated within a microparticle (Fig. 1).

The nanoparticle component displays maximum surface area that increases the drug dissolution rate and the bioavailability after oral administration, while the microparticle one allows the loading of high drug cargos and the spatiotemporal fine-tuning of the release in the gastrointestinal tract. In addition, the use of mucoadhesive polymers for the production of the micrometric component would prolong the residence time of the NiMDS in the gut, maximizing the intestinal absorption of the drug and likely enabling a reduction in the frequency of administration. Aiming to improve the biopharmaceutical performance of poorly water-soluble drugs that demand administration several times a day, my research group investigates NiMDSs for the controlled release of antiretrovirals by the oral route to optimize the anti-HIV therapy. To achieve this, an innovative simplified two-component NiMDS comprised of pure nanoparticles of the poorly water-soluble drugs are encapsulated within film-coated mucoadhesive microparticles. This strategy is especially appealing for poorly water-soluble drugs that do not undergo fast dissolution in the gastrointestinal medium and where the dissolution rate is capitalized to control the release of the drug from the carrier. At the same time, it could be extended to more soluble drugs with the appropriate film-coating of the microparticle surface with a water-insoluble and permeable membrane that controls the release kinetics. In this presentation, I will discuss the concept and overview the most recent results employing this delivery approach.
Chun Xia Zhao
The University of Queensland, Australia
Title: Platform technologies for accelerating the translation of nanomedicine

Biography:
Chun-Xia Zhao is an Australian Research Council (ARC) Future Fellow and Associate group leader at Australian Institute for Bioengineering and Nanotechnology at The University of Queensland, Australia. She leads a research team with a focus on bio-inspired engineering and microfluidics. She has been focusing on innovative research as evidenced by her four patents. Dr Zhao’s research has attracted more than $2.5 M in research funding since 2011, including four Australian Research Council projects as the lead investigator, two national prestigious fellowship, and six UQ grants. Dr Zhao has been recognised for scientific excellence with a 2016 UQ Foundation Research Excellence Award. She has built extensive collaborations with scientists at top universities such as Harvard University, Cornell University, etc. She was invited to visit Harvard University as a Fellow of the School of Engineering and Applied Science. She also serves as the Editor-in-Chief, Editorial Board member for several journals.
Abstract:
Cancer remains a leading cause of death worldwide. Approximately 8.2 million people died from cancer in 2012, and new cancer diagnoses are expected to increase from 14 million in 2012 to 22 million within the next two decades. Multifunctional nanoparticles hold tremendous promise for cancer diagnosis and treatment, by incorporating targeting, stimuli-responsive release and imaging functions to achieve targeted delivery, controlled release and real-time diagnosis. They have attracted significant interest and undergone an explosive growth over the last decade. However, the majority of nanoparticles have not reached the clinic due to two main barriers: (1) more functionality requires more synthetic steps, resulting in not only difficulty in reproducibly synthesising nanoparticles having consistent properties, but also low yields and high production cost and high probability of failure during synthesis; (2) high complexity means more convoluted behaviour and effects in vivo, difficulties in understanding interactions between nanoparticles and biological systems. To address these fundamental issues, my lab has been focusing on the development of facile one-step or one-pot approaches for producing multifunctional nanoparticles for targeted drug delivery. A combinatorial multifunctional liposomes and polymeric nanoparticles have been fabricated with well controlled properties including particle sizes, zeta potential, targeting ligand density, etc., using a robust and reproducible one-step method. Their biological functions were systematically investigated to screen the optimal formulation having the best tumor specificity using in vitro 2D monolayer cell culture and 3D tumor spheroids model in comparison with in vivo experiments. On the other hand, we have also developed in vivo mimicking chips to speed up the evaluation of the library of nanoparticles.
Ying Wang
Caner Research Institute, Southern Medical University, China
Title: Targeted nanoparticles was involved in overcoming MDR induced by heterogenous cancer cells

Biography:
Ying Wang is the Associated Professor in cancer research institute at Southern Medical University in China. Her research interest is broadly in biomolecular engineering and nanotechnology and biological mechanism of nanoparticles, with a focus on drug delivery system for cancer therapy, antiangiogenesis therapy, cancer molecular imaging, natural plant nanocarrier. Her research program is currently supported by Natural Science Foundation of China and Natural Science Foundation of Guangdong Province.
Abstract:
Heterogenous cancer cells possess cancer multidrug resistance (MDR) due to their relative quiescence and ABC-transporter expression. Heterogenous cancer cells can be detected by an Rh123 exclusion assay for identifying Rh123low population. Several attempts to explore nanoparticles (NPs) based strategies have involved overcoming cancer MDR due to their ability for bypassing MDR drug-efflux pump. Targeted NPs have been shown to have the potential to reverse MDR-mediated drug efflux, indicating active targeted effect and endocytic effect of NPs which correlates with the drug-efflux pump mechanism play a key role in overcoming MDR. In the present study, we fabricated targeted nanoparticles entrapped with Rh123 (Rh123 NPs) to investigate the endocytic effect and targeted effect of these nanoparticles on heterogeneous Rh123low population.

We found the Rh123low population stained by Rh123 NPs exhibited similar heterogeneity to that stained by Rh123. In addition, the ABC-transporters of Rh123low population did not contribute to the uptake of free Rh123 or Rh123 NPs. Interestingly, ABC-transporters in the Rh123low population stained by Rh123 were possibly responsible for Rh123 efflux, while Rh123 NPs were not susceptible to ABC-transporters in the Rh123low population. It was explained that the synergistic effect of the targeted NPs was beneficial for Rh123 NPs to avoid the dye-efflux pump in heterogenous cancer cells. Compared with the individual endocytic effect, the targeted effect played more important role for overcoming the drug efflux pump of the ABC-transporter in heterogenous cells.
Beatriz P. Nobre
Lisbon University, Portugal.
Title: Supercritical micronization as an important technique to produce pharmaceutical microparticles

Biography:
Beatriz P. Nobre has expertise in supercritical fluids, namely on supercritical fluid extraction of bioactive compounds from microalgae and vegetable matrices and on micronization of pharmaceutical compounds.. She is a researcher at the Centro de Química Estrutural of Lisbon University and also at the Bioenergy Unit of the National Laboratory of Energy and Geology. She has participated in several national and international research projects in the biotechnology and chemical engineering fields.
Abstract:
Particle size of pharmaceuticals compounds can play a significant role in the amount of the active principle absorbed by the human body and with many compounds it is possible to provide dosages well below the toxicity threshold. Supercritical fluid anti-solvent processes (SAS) were recently proposed as alternative to liquid anti-solvent ones. The SAS process works similarly, but instead of the use of a liquid solvent, in which the compound to be micronized is insoluble, it is used a supercritical fluid. The combination of the high solvent power of supercritical fluids to dissolve the organic solvent and the low solubility of the pharmaceutical compounds in the supercritical fluids makes this technique the most suitable for the precipitation of pharmaceutical compounds. On the other hand, it is possible to recover the supercritical anti-solvent by simple decompression, avoiding complex treatments typical of the liquid process. Supercritical CO2 is the most used antisolvent in SAS processes. In addition to the advantage of replacing toxic solvents, CO2 has also the capability of producing pure particles with special morphologies. Moreover, a wide range of compounds can be processed using this solvent. SAS micronization of pharmaceuticals and bioactives, such as sodium fusidate (figure 1), fusidic acid and astaxanthin, has been carried out in our laboratories. Nano and micro particles with morphology and particle size suitable for its use in pharmaceutic formulations were obtained. The most interesting results of these studies will be discussed, with special focus on the effect of SAS operation parameters in the particle size and particle size distribution.
Sandeep Palvai
Indian Institute of Science Education and Research (IISER)-Pune, India
Title: Targeting Phosphatidylinositol-3-Kinase Signaling with Simultaneous DNA Damage in Cancer Cells by using Cholesterol based Chimeric Nanoparticle

Biography:
Sandeep Palvai has developed a passion for interdisciplinary approaches towards formulating next generation nanomedicine to treat cancer. After graduating with an M.Sc in organic chemistry, he has joined in Dr. Sudita Basu Lab, Indian Institute of Science Education and Research, Pune, India. During his career as a Ph.D. student he has gained expertise in formulating lipidic and polymeric nanoparticles to co-deliver various anticancer drugs to cancer cells and also evaluating their in-vitro biological activity. One of his research highlights is delivering multiple drugs to kill cancer cells more efficiently by using biocompatible chimeric nanoparticle. He has published thee peer reviewed international publication, one book chapter and also shared authorship in two publications. His enthusiasm helps him to keep connected with people by attending several conferences and workshops. After graduating, Sandeep hopes to expand and pursue his research career in developing nanomedicine with cutting-edge techniques to treat several other diseases including cancer.
Abstract:
Phosphatidylinositol-3-kinase (PI3K) signaling has been a major target as it is found to be mutated and over expressed in many types of cancers. PI3K inhibition alone by small molecules failed to offer effective therapy since drug resistance has been a major problem. Recent studies reveal that inhibiting singling pathways with simultaneous DNA damage is effective to combat cancer. However, targeting PI3K signaling with small molecules in combination with DNA damaging drugs would be challenging as it leads to severe side effects due to nonspecific interactions in cancer patients. To this end, we have developed a biocompatible, biodegradable cholesterol-based chimeric nanoparticle (CNP), which can simultaneously load PI103, cisplatin and doxorubicin in a controlled ratiometric manner. Size, shape, and morphology of these CNPs were characterized by dynamic light scattering (DLS), ï¬eld-emission scanning electron microscopy (FESEM), atomic force microscopy (AFM), and transmission electron microscopy (TEM). CNPs demonstrated increased release of PI103, doxorubicin, and cisplatin through sustainable manner over 120 h at pH = 5.5 compared to neutral pH. The CNPs showed much improved in vitro cytotoxicity in HeLa,
HL60, MCF7, and MDA-MB-231 cancer cells compared to a free drug cocktail at 24 and 48 h also induced apoptosis. Confocal laser scanning microscopy (CLSM) imaging revealed that these CNPs were internalized into cells through endocytosis in a time dependent mode over 6 h and retained inside for 48 h in HeLa, MDA-MB-231, and MCF7 cells. These CNPs exhibited their efficacy by damaging DNA and inhibiting Akt as a downstream modulator of PI3K signaling in HeLa cervical cancer cells. To conclude, these CNPs have the potential to open up new directions in next-generation nanomedicine by targeting multiple oncogenic signaling pathways with simultaneous induction of DNA damage for the augmented therapeutic outcome by reducing off-target toxicity and overcoming drug resistance.
Chia-Hua Lin
National Formosa University, Taiwan
Title: Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes
Biography:
Chia-Hua Lin has his expertise in nanotoxicology, environmental toxicology and nanobiotechnology. His group consists of multidisciplinary team of researchers including chemist, engineers and cell and molecular biologists. He has established platforms for evaluating toxicity of nanoparticles and environmental pollutants in vivo and in vitro, including characterization, tissue distribution, metabolism, biochemical responses and molecular mechanisms. He has a sub-specialization in developing engineered biomaterials for cancer therapy, and investigating their effect on in vitro and in vivo photo-therapeutic evaluation.
Abstract:
Statement of the Problem: Because of their wide range of unique properties, graphene and grapheme family nanomaterials (GFNs), isolated from their three dimensional parental material graphite, can serve as starting materials for developing innovative therapeutic strategies. In medicine, GFNs have emerged as the most promising seed for various applications, such as drug delivery, anticancer therapy, and biosensing. However, the toxicity and safety of GFNs in the treatment of human diseases should be systematically assessed both in vitro and in vivo. The present study aimed to consecutively assess the immunotoxicity of graphene oxide nanoplatelets (GONPs) and reduced GONPs (rGONPs) on THP-1 cells, a human acute monocytic leukemia cell line. GONPs induced the expression of antioxidative enzymes and inflammatory factors, whereas rGONPs had substantially higher cellular uptake rate, higher levels of NF-κB expression. These distinct toxic mechanisms were observed because the two nanomaterials differ in their oxidation state, which imparts different affinities for the cell membrane. Because GONPs have a higher cell membrane affinity and higher impact on membrane proteins compared with rGONPs, macrophages (THP-1a) derived from GONPs treated THP-1cells showed a severer effect on phagocytosis. By consecutive evaluation the effects of GONPs and rGONPs on THP-1 and THP-1a, we demonstrated that their surface oxidation states may cause GFNs to behave differently and cause different immunotoxic effects.
Nor Azah Yusof
Universiti Putra Malaysia, Malaysia
Title: Folic Acid-Conjugated Chitosan-Based Quantum Dot System For Cancer Cell Imaging And Therapy

Biography:
Nor Azah Yusof She started working on optical chemical sensor for toxic metal detection. She further developed her expertise on electrochemical based biosensor. She has been working on DNA based biosensor and protein based biosensor. Further information can be obtained from our group website www.upmbiosensor.com. She wish to further develop her electrochemical reader and biochip that she has started working on for 5 years into a prototype and later be commercialized. She is seeking partner to together develop the technology into a suitable kit and further commercialized the diagnostic kit for home use. She also work on molecular imprinted polymer and currently working with some industrial partner to develop sorbent to remove contaminants from water.
Abstract:
Current research in targeted delivery through engineered nanocarries has demonstrated a promising mild stone in achieving specialised and personalised medicine following the exploitation of biomolecules uniquely expressed by cancer cells to conveniently transport therapeutics with improved efficiency and limited systemic toxicity. Here, we report the synthesis of a nanocomposite, semiconductor quantum dots (QDs) system following simple wets chemistry method for cancer theranostic applications.

The chemistry involves the loading of an anti-cancer drug (5-Fluororacil) into Mn-ZnS QDs encapsulated chitosan (CS) biopolymer with folic acid (FA) conjugation. The binding of the QDs to FA conjugated CS was confirmed using XPS, FTIR and EDX analysis and the elemental mapping of the drug loaded nanocomposites was characterised using FTIR and DSC analysis. The cytotoxicity analysis performed on breast cancer cells (MCF-7 and MDA cell lines) and normal breast cell lines (MCF-10) shows the effectiveness of the sample towards the destruction of cancer cells and its non-toxicity towards normal cells. The mechanistic study by means of flow cytometry revealed that the drug loaded conjugates has high selectivity towards the destruction of cancer cells than bare anti-cancer drug. The results demonstrated that the decoration of FA on the drug loaded conjugates owing to its stronger affinity toward folate receptors largely expressed by cancerous cells enhances the level of drug uptake by the cells and the QDs incorporated into the system allowed selective imaging of the cancer cells under confocal microscopy. The results highlight the suitability of FA-conjugated Mn-ZnS QDs as promising probe for cancer theranostic.
R.M. Gamini Rajapakse
University of Peradeniya, Sri Lanka and Imperial College, London
Title: Synthesis, characterization and release kinetics of target-specific, porous nanoparticle- encapsulated anticancer drugs

Biography:
R.M. Gamini Rajapakse (B.Sc. Honors, Ph.D., D.I.C, FNASSL) is a Senior Professor in Chemistry attached to the University of Peradeniya, Sri Lanka and is the Coordinator of Nanotechnology Degree Programs. He has diverse research interests in versatile areas of Nanotechnology and is currently supervising 15 Ph.D. students. He has served in many universities in UK, USA and Japan and earned over 100 indexed publications, large number of communications and 15 National Awards for his research. Targeted drug delivery is one of his fascinating research areas where he has trained several students in this area of research and produced indexed publications in reputed international journals.
Abstract:
The pH-sensitive drug delivery systems can achieve targeted drug delivery and systemic control release. The purpose of this research is to synthesize composite formulae comprising of anticancer drugs such as bis(8-hydroxyquinoline), cisplatin, doxorubicin etc. encapsulated in biocompatible and non-toxic porous inorganic nanoparticles, characterize them and to study the drug loading capacities and slow-release kinetics the latter triggered by the pH of the medium. We have successfully synthesized porous nanoparticles of both calcium carbonate, magenesium hydroxide and hydroxyapatite through soft-templated and molecular-templated bottom up approaches and the above anticancer drugs were separately encapsulated in them. Over 30% drug loading capacity and over 80% encapsulation efficiency were obtained for all drugs. The materials synthesized were extensively characterized by XRD, FT-IR, SEM and XRF techniques and the drug encapsulation in porous nanoparticles confirmed. Drug release kinetics was investigated in aqueous media at different pH values and found that favorable slow release kinetics is obtained at slightly acidic pH values typical of those of cancer cells.

There is hardly any release of the drugs at neutral or slightly basic pH values typical of those of blood plasma and healthy cell fluids. As such, advancing pH-sensitive drug delivery systems can make a significant improvement in cancer chemotherapy by reducing both cytotoxicity of drugs to healthy cells and drug dosage requirements while increasing the bioavailability and efficacy of the drugs
Oluwatobi S Oluwafemi
University of Johannesburg, South Africa
Title: Synthesis, antibacterial, cytotoxicity and sensing properties of starch-capped silver nanoparticles

Biography:
SO Oluwafemi is a National Research Foundation (NRF), South Africa rated researcher at the department of Applied Chemistry, University of Johannesburg. His research is in the broad area of nanotechnology and include green synthesis of semiconductor and metal nanomaterials for different applications which include but not limited to biological (Imaging, labeling, therapeutic), optical, environmental and water treatment. He has author and co-author many journal publications, book chapter and books. He is a reviewer for many international journals in the field of nanotechnology and has won many accolades both local and international.
Abstract:
We herein report the antibacterial, cytotoxicity and sensing properties of dextrosed reduced starch-capped silver nanoparticles (Ag-NPs). The synthesis involves the use of water, starch, and dextrose which are environmentally benign materials as the solvent, stabilizing, and reducing agent respectively while AgNO3 was used as the silver precursor. The reaction was carried out without the use of any accelerator and the antibacterial activities of the Ag-NPs synthesised at different reaction time were investigated. The as-synthesised Ag-NPs were characterized using UV-vis absorption spectroscopy, Fourier transform infra-red spectroscopy (FTIR), Raman spectroscopy, X-Ray diffraction analysis (XRD) and High resolution transmission electron Microscopy (HR-TEM). Antibacterial study show that, all the as-synthesised Ag-NPs show good antibacterial activities against E coli and two strains of P. aeruginosa, which are antibiotic sensitive and resistant bacteria. In addition, the Ag-NPs produced at longer reaction time (48 h) showed a better antibacterial efficacy than those synthesised at lower reaction time. The cytotoxicity evaluation on Human THP-1 monocyte cell line indicated that the as-synthesised Ag-NPs are less toxic than AgNO3 at lower concentrations. The as-synthesised Ag-NPs are very sensitive towards hydrogen peroxide (H2O2) and show a linear response over a wide concentration range upto 10−10 M H2O2
Suhaili Shamsi
Universiti Putra Malaysia, Malaysia
Title: NanoCUR: formulation and its effect on major human cytochrome P450 enzymes
Biography:
Dr Suhaili Shamsi graduated from the University of Western Australia, Australia with a PhD after completing her Bachelor of Science with First Class Honours in Biochemistry at Universiti Putra Malaysia. Her PhD thesis is entitled “Development and evaluation of curcumin-loaded Pluronic F127 nanoformulation” that focuses on the synthesis of a simple, cost-effective yet stable curcumin nanoformulation that maintained most of the biological activities of curcumin. Dr Suhaili Shamsi is currently a senior lecturer at the Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia. Her current research interest focuses on incorporating nanotechnology into the development of adjuvant, specifically for chemotherapy purpose, by utilizing the myriad of natural products found locally in Malaysia. Apart from that, Dr Suhaili Shamsi is also interested in studying the mechanism of drug-drug/food interactions through the modulation of protein efflux transporters and cytochrome P450 enzymes.
Abstract:
Statement of the Problem: Curcumin (CUR) is a phenolic compound from turmeric with multiple beneficial pharmacological properties, many of which result from enzyme modulation. CUR is a potent inhibitor of the cytochrome P450 (CYP) family of enzymes that play a vital role in the metabolism of drugs, carcinogens and toxins. To improve its in vivo solubility, stability and bioavailability, CUR has been formulated into many types of nano-sized formulations. However, it is not known whether the transformation of CUR into nanoformulations would affect its enzyme activities or not. The aim of this study was to compare the cellular response and CYP enzyme inhibitory activities of CUR before and after its encapsulation in a nanocarrier. Methodology & Theoretical Orientation: A micellar formulation of CUR (NanoCUR) was developed using thin film hydration method with Pluronic F127 as stabilizer. To determine particle integrity after processing, the mean size of NanoCUR was monitored by dynamic light scattering (DLS). Cytotoxicity, anti-cytoproliferative effect and cellular uptake of NanoCUR were evaluated in the human liver HepG2 cell line. The effects of NanoCUR on CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in human recombinant enzyme systems and HepG2 cells were evaluated. Findings: NanoCUR was amenable to lyophilisation, and retained its nano-sized spherical dimensions (17 – 33 nm) upon dilution with HBSS and EMEM. NanoCUR was a weaker cytotoxic agent against the HepG2 cells compared to CUR in solution (sCUR). This was due to a lower cellular uptake of CUR from NanoCUR than from sCUR. NanoCUR was a weaker inhibitor of CYP2C9 and CYP2D6 than sCUR. NanoCUR was, however, 1.76-fold more potent against the CYP3A4 (IC50 5.13 ± 0.91 µM) metabolic function. Both sCUR and NanoCUR had no effect on the CYP3A4 mRNA levels in the HepG2 cells. Conclusion & significance: NanoCUR possessed the biological activities of CUR, albeit at a lower potency and response rate. The Pluronic F127 micellar structures in NanoCUR may impart synergism to the CUR-mediated inhibition of CYP3A4 metabolic function, which is an important consideration for further development and evaluation of NanoCUR as a potential adjuvant for anti-cancer chemotherapy.
Chen Hsu
Lungwha University,Taiwan
Title: Fabrication, characteristic, and future-biomedicine applications of villous oxalates

Biography:
Chen Hsu, D. Phil, now is a Professor of Department of Chemical and Materials Engineering, Editor of Nanomedicine & Nanotechnology Open Access, Editorial member of SCIRES Journal of materials, Head of the Semiconductor and Ceramic Material Laboratory, Member of Chinese Material Science Association, Member of ROC Micro Systems and Nanotechnology Association, Member of Chinese National Defense Science and Technology Association, and Member of ROC Ministry of Economic Affairs. Chen Hsu received his D. Phil. degree from Oxford University in Materials in 1999. Dr. Chen Hsu and coworkers have done many interesting research works on, such as, biosensing devices for biosensor in vitro…Currently Prof. Chen Hsu’ researches partially focus on biosensors, nanomedicines, and novel drug delivery systems without using superpararmagnetic nanoparticles.... The especial research aims are concentrated on microstructures to applications.
Abstract:
Statement of the Problem: Villous zinc oxalate (ZnC2O4) is a kind of phase-refined materials. It can be prepared using various methods such as solid-phase, liquid-phase, or so-gel methods. Zinc acetate (C4H6O4Zn) and oxalic acid are commonly used to prepare ZnC2O4. ZnC2O4 can be used to preparing by the sol-gel method from zinc oxide and graphene. In reverse, ZnC2O4 can be used to prepare as ZnO for cancer therapy. However, the use of ZnC2O4 on human body is not clear for the time being. The reason could be this material is belongs to paramagnetic materials and cannot be used on biomedicine along. Villous ZnC2O4 was successfully prepared from reduced graphene oxide (RGO) and zinc oxide (ZnO) using a two-precursor sol-gel method, and scanned using a matrix-dotted microbeam laser. Application of the laser caused the synthesized ZnC2O4 to melt and resolidify, forming uniformly spread nanovillous protrusions. The characteristics of the microstructures were examined using X-ray diffraction, energy dispersive X-ray analysis, field emission scanning electron microscopy, and Raman spectroscopy. Villous ZnC2O4 is open structure and can dissolve in liquid. Moreover, it can be attracted through impurity sites, resulting in oversaturation. There, it can be used on biomedicine applications, such as; it can be a surface coating materials on superparamagnetic nanoparticles as targeted medicine. It can also be used as a targeted substrate for other medicine coating or interstial in there. This targeted goal can be achieved by the novel drug delivery system for non-superparamagnetic nanoparticles and is under developing in our group.
Shashiprabha P. Dunuweera
University of Peradeniya, Sri Lanka
Title: Encapsulation of Anticancer Drug Cisplatin in Porous Vaterite Nanoparticles for Targeted Delivery and Slow Release

Biography:
Shashiprabha Dunuweera (B.Sc. Honors) is an emerging scientist pursuing research studies leading to effective and efficient methods to targeted delivery of anticancer drugs in order to suppress the cytotoxicities of the drugs to other cells while increasing their efficacy and decreasing the dosage requirements. Although he is still at the beginning of his research career, through his extraordinary dedication, he has developed expertise in synthesizing safe and efficient drug carriers based on porous nanoparticles for encapsulating anticancer drugs for safe targeting and controlled release of the drug triggered by the acidity of the cancer cells. He has participated actively in a number of international conferences presenting his research findings and educating fellow scientists and has already earned a few journal publications at the age of 26 years. Due to his characteristic kindness to every living being, Shashiprabha has devoted his energy and time towards combatting cancers by developing safer chemotherapy.
Abstract:
Statement of the Problem: Cis-platin is a commonly used anticancer drug which is the first of such platinum-based anticancer drugs developed. The cis configuration enables the binding of the coordination complex to two DNA strands and thereby crosslinking the DNA strands triggering the cells to die in a programmed manner. Cisplatin is administered to patients intravenously as a short-term infusion in normal saline for treatment of solid malignancies. It is used to treat various types of cancers which include sarcomas and some carcinomas such as small cell lung cancer, ovarian cancer, lymphomas, bladder cancer, cervical cancer, and germ cell tumors. It is found that cisplatin is particularly effective against testicular cancer with a cure rate of 10-85%. However, cisplatin is associated with numerous side effects which include nephrotoxicity, neurotoxicity, nausea and vomiting, ototoxicity (hearing loss), electrolyte disturbance and haemolytic anemia.
Most of these side effects can be either reduced or overcome if cisplatin could be encapsulated in a suitable host material and directed towards cancer cells targeted manner and allowed to release only in minimum sufficient dose in uniform manner. In order to accomplish these targets, we have prepared hollow nanoparticles of calcium carbonate and encapsulate cisplatin in them and studied their release kinetics in buffered solutions of defined pH values. Since cancerous cells are more acidic (pH range 5.00 – 6.00) compared to normal cells (pH range 7.00 -8.00), and that calcium carbonate is stable in neutral pH media while decompose slowly in low acidic conditions, the choice of calcium carbonate nano-carrier host is highly justified for targeting the encapsulated drug to cancerous cells for slow-release of the drug only in the vicinity of the cancer cells.
Abbas Teimouria
Payame Noor University,Iran
Title: Fabrication and characterization of novel silk /chitin hydrogel /MCM-41 composite scaffolds for tissue engineering
Biography:
Abbas Teimouria,Payame Noor University,Iran
Abstract:
Tissue engineering has recently developed a growing field in the regeneration of tissues Biomaterials are required in tissue engineering techniques for the construction of scaffolds by which relevant cells can be attached, grown, and proliferated. Silk fibroin (SF) is a natural polymer produced by a variety of insects. Recently, silk cocoons from Bombyx mori have been used as an in-access material for tissue regeneration purposes [1].
Chitin, which is known to be a biocompatible and biodegradable polysaccharide, is the most abundant polymer on earth after cellulose. It is fabricated from the shells of shrimp and squid pens. It has also been found that the chitin hydrogel could be prepared by using this solvent system. In recent studies, the calcium solvent system has been found to be a suitable solvent to dissolve the chitin in the mild conditions [2].
silica exhibits several properties associated nwith an ideal material for grafting and scaffolding [3]. The nano ranged bioactive silica particles having large surface area canform a tighter interface with the polymer matrix in composites. Nano-bioglass ceramic particles possessing silica as its one of the chief contents not only provide polymer scaffolds with biomineralization
capability but also increase the stiffness of polymer material without greatly decreasing the mechanical strength [4].
In continuation of our previous studies on the construction of composite scaffolds [5-6], In this research, novel porous composite scaffolds consisting of silk, chitin, and MCM-41 were prepared using the freeze-drying method. The prepared nanocomposite scaffolds were characterized by SEM, XRD, BET, TGA and FT-IR techniques. In addition, swelling, degradation and biomineralization capability, cell viability and cell attachment of the composite scaffolds were evaluated.

Biography:
Dr. Muhammad Abid Sheikh is currently working in Department of Microbiology and Molecular Genetics, University of the Punjab as Assistant Professor.
Abstract:
Parkinson's Disease (PD) is a chronic neurodegenerative disorder characterized by motor deficits which result from the progressive loss of dopaminergic neurons. Gene therapy using growth factors such as VEGF seems to be a viable approach for potential therapeutic treatment of PD. In this study, we utilized a novel non-viral gene carrier designated as PEI-PLL synthesized by our laboratory to deliver VEGF gene to study its effect by using both cell culture as well as animal models of PD. For cell culture experiments, we utilized 6-hydroxydopamine (6-OHDA) mediated cell death model of MN9D cells following transfection with either a control plasmid or VEGF expressing plasmid. As compared to control transfected cells,
PEI-PLL mediated VEGF gene delivery to MN9D cells resulted in increased cell viability, increase in the number of Tyrosine hydroxylase (TH) positive cells and decreased apoptosis following 6-OHDA insult. Next, we studied the therapeutic potential of PEI-PLL mediated VEGF gene delivery in SNPc by using unilateral 6-OHDA Medial forebrain bundle (MFB) lesion model of PD in rats. VEGF administration prevented the loss of motor functions induced by 6-OHDA as determined by behavior analysis. Similarly, VEGF inhibited the 6-OHDA mediated loss of DA neurons in Substantia Nigra Pars Compacta (SNPc) as well as DA nerve fibers in striatum as determined by TH immunostaining. In addition, PEI-PLL mediated VEGF gene delivery also prevented apoptosis and microglial activation in PD rat models. Together, these results clearly demonstrated the beneficial effects of PEI-PLL mediated VEGF gene delivery on dopaminergic system in both cell culture and animal models of PD.
Biography:
Developed and evaluated niosomes a kind of nano-vesicular surfactant based drug delivery system for diabetic drug and it was found to be promising and effective drug delivery system. Research paper was published in Journal of Young Pharmacist in 2010. Associated with many projects related to vesicular systems and published few papers in reputed journals. February 2013. Reviewed project for Nano and Advanced materials Institute Limited (NAMI), Hong Kong, under 13th round of the subject “Development of nano-vesicular delivery systems for use in skincare products.” Won the honorarium of 128 USD. Beside this reviewed more than 20 research papers for various reputed journals like Elsevier, Bentham Science and Pubmed Index Journals. Editorial Board member of various reputed journals like Journal of Pharmaceutical Technology and Drug Research, International Research Journal of Pharmacy, Advances in Biomedicine and Pharmacy: Biomedicine Journal etc. Assisted graduate students performing projects on various nano vesicular systems like liposomes, niosomes, nano lipid carrier (NLCs). Executed doctoral research activity with students and research scholars. Developed stimuli-sensitive hydrogels and nano vesicular systems for the treatment of Glaucoma. Successfully completed project on NLCs and developed formulation for cosmetic skin care. Various papers were published in reputed impact factor journals. Recently published work in European Journal of Pharmaceutics and Biopharmaceutics in impact factor 3.976 could be the milestone in the field of cosmetic skin care and nano technology. Mentored post graduate students conducting research projects. Till date 13 projects were completed and 1 Ph.D research project going to commence. More than 37 Publications were accomplished with over 180 citations. Presented research paper in various conferences and seminars. Most recently bagged best oral presentation award in World congress in drug discovery and development 2016, held at Indian Institute of Science, Bengaluru, India.
Abstract:
The purpose of this study was to prepare and evaluate Carboplatin loaded polymeric magnetic microspheres for specific targeting. Methodology: It was prepared by emulsion-solvent evaporation method and evaluated with respect to particle size analysis, entrapment efficiency, IR studies, DSC, Swelling kinetics, magnetite content, in vitro magnetic responsiveness in a 0.21 kgauss magnetic field, in vitro drug release studies, in vivo drug targeting studies and stability studies. Results & Discussions: Spherical particles of average 169.3 nm to 285 nm in diameter and incorporation efficiency up to 77.91% were obtained. By chemical analysis, total percentage of Fe2O3 in the microspheres was found between 69 % - 96.68 %. Cumulative percent drug release after 8 hours were 99.31%, 98.89%, 99.47% for F-1 to F-3 MMS and after 24 hours 98.755 %, 98.33 %, 99.93 % was found for F-4-F-6 MMS respectively.
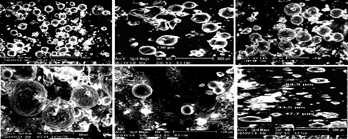
Conclusion: Results of in vitro magnetic responsiveness and in vivo targeting demonstrated that the retention of microspheres in presence of magnetic field was significantly more than those in the absence of the magnetic field. Stability studies showed that maximum drug content was found in the samples stored at 4°C. This study was a satisfactory preliminary attempt in development of magnetically modulated novel parenteral carrier for CPt. However magnetic field strength and gradient, distribution of the magnetite in the microspheres, application time of the magnetic field and in vitro in vivo co- relationship under diseased state are the most likely parameters which need to be optimized for the successful use of this delivery system in localized drug therapy.
Manohar Balaraman
CSIR-Central Food technological research Institute, India
Title: Nano-liposomes using supercritical fluid system for nutraceuticals

Biography:
Manohar Balaraman, currently Chief Scientist & Head at Department of Food Engineering, CSIR-Central Food Technological Research Institute, Mysore, India, has about 33 years of experience in the area of food process engineering. He has 72 research publications in peer-reviewed international journals, 50 presentations in national and international conferences, 2 book chapters. His major research interests include supercritical fluid extraction, molecular distillation and biotechnological approaches to extract bio-actives from natural materials. He has visited Korea and China. He is a recipient of UNIDO fellowship during 1991-92. He is a life member of Indian Institute of Chemical Engineers, Association of Food Scientists & Technologists.
Abstract:
The liposomes as bioactive carriers are getting attention worldwide because of its unique stability against severe environmental, chemical changes with improved solubility and increased bioavailability. There are several methods for preparation of liposomes such as thin film evaporation, ultrasonication, high pressure homogenizer, membrane processing and supercritical CO2. Most of the techniques of encapsulation process involve use of harmful solvents and high temperature treatment which directly affects both quality and quantity of nutraceutical components.
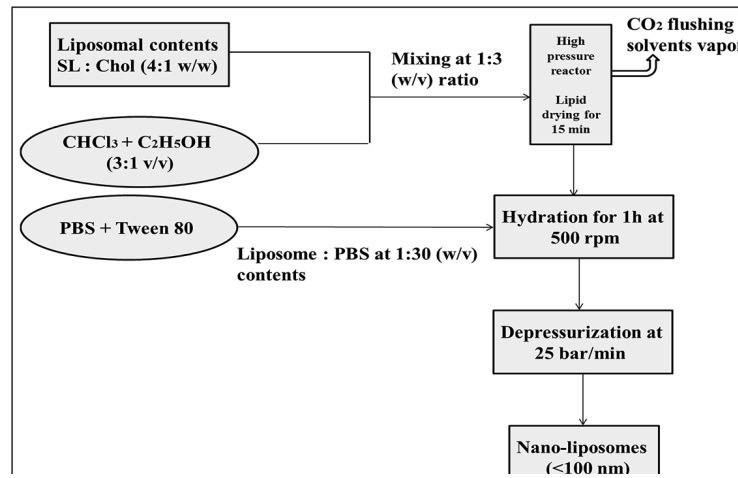
The supercritical (SC) CO2 method of liposomes preparation works on the principle of Gas anti−solvent technique (GAS) which is a novel approach to produce nano-liposomes of superior characteristics in a single step. The SC-GAS method of liposomes production is more acceptable because of its favorable mild conditions and its nontoxic nature which would favor encapsulation of thermo sensitive compounds. The major advantages of this process are negligible solvent residue in final product, moderate temperature processing which prevents heat sensitive product from degradation, time saving process which involves only mixing liposome ingredients followed by depressurization, size of vesicles can be tuned with variation in depressurization rates. Many applications of GAS technique for nutraceuticals shall be briefed that include delivery of tea polyphenols in the form of nanoliposomes.
Samira Jafari
Imam Khomeini International University, Iran
Title: Nanotechnology-based Combinational drug delivery systems for breast cancer treatment

Biography:
Samira Jafari received her master’s degree in analytical chemistry from the University of Tabriz and is currently pursuing her doctorate of chemistry in the analytical area at Imam Khomeini International University. In addition to her master’s degree, Samira is well travelled in her schooling and as such has acquired a wide range of different chemistry styles. With this experience, she gleaned and culminated a wide scope of techniques to develop a novel method for targeting various cancers efficiently with relatively low costs as compared to customized patient medicines. With a generic customized cancer drug delivery system as described in her work, a new field of focus is presented that can make large strides in the fight against breast cancer
Abstract:
Breast cancer is arguably the most common cancer faced by females today and the second most common cause of death in women in the world. Indeed, this illness has garnered much attention in the field of pharmacology research. Modern chemotherapeutic anticancer treatments have come a long way in the fight against breast cancer, thus bringing science closer to a cure. However, the nature of these drugs is to attack both cancerous and non-cancerous cells at the same time. With this current approach, a patient’s health, in addition to the cancer, can succumb to chemotherapies. To counter this problem, and increase the efficacy of cancer treatment, methods to customize therapeutic anti-cancer drugs have emerged in the form of targeted drug delivery systems. In our studies, we present a method of a drug delivery using magnetic polyurethane. Here, we describe a biocompatible magnetic polymer that can be used to direct chemotherapeutic drugs to cancerous regions in a body using an external magnet. We show how a co precipitation method with magnetic nano-particles (MNPs) followed by a silica coating process and an in situ polymerization yields the magnetic polyurethanes used in this study. Verification of synthesis for the drug carrier is shown using the characterization techniques of scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), and a vibrating sample magnetometer (VSM). The efficiency with drug loading and release of chemotherapeutic medications to the synthesized magnetic polyurethanes is monitored using an HPLC-UV detector. Our findings present a new biocompatible drug delivery system with a high capacity for loading and directing tow various chemotherapeutic drugs simultaneously to cancer sites with little to no toxicity to the surrounding non-cancerous cells.
Satish Agrawal
CSIR-Central Drug Research Institute, India
Title: CD44 targeting hyaluronic acid coated lapatinib nanocrystals for fostered efficacy against triple-negative breast cancer
Biography:
Agrawal Satish has his expertise in formulation of novel, specially nano-drug delivery systems and their biomedical testing in animal models. He has completed his B.Pharm from Pune University, M.Pharm from BITS Pilani University, and currently pursing PhD from Central Drug Research Institute, Lucknow, India. He has also completed PGDBA (Operations) from Symbiosis centre for Distance Learning, Pune. He has published 9 papers in reputed journals and has strong passion for research in drug delivery systems.
Abstract:
Lapatinib (LPT) is an orally administered drug for the treatment of metastatic breast cancer. However, there are many drawbacks associated with LPT that limits its therapeutic activity. In order to address the limitations associated with LPT, we have prepared its nanocrystals (LPT-NCs) those were subsequently coated with hyaluronic acid (HA) to produce LPT-HA-NCs. The detailed investigation of LPT-HA-NCs showed the superior anticancer activity than its counterparts LPT-NCs and free LPT. LPT-HA-NCs were associated with increased cytotoxicity and the enhanced uptake in MDA-MB-231 cells due to active targeting to CD44 and EGFR receptors. It was also evident that the LPT-HA-NCs induced apoptosis is due to the Fas and mitochondrial membrane potential dependent pathway. The results of in-vitro studies were followed very well in the in-vivo studies. In the triple negative 4T1 cells induced breast tumor bearing female Balb/C mice; LPT-HA-NCs not only increased the residence time of LPT, but also targeted the tumor, reduced the tumor burden, and increased overall survival. Our findings clearly suggest that HA coated LPT-NCs formulation enhances the activity of LPT against triple negative breast cancer. It exhibited magnificent therapeutic outcome at low dose thus presenting a strategy to reduce dose administrations and minimize dose related toxicity.
Md Abdus Subhan
Shah Jalal University of Science and Technology,Bangladesh
Title: Biological and Micellar Properties of Nano Transition Metal (Co, Fe, Ni, Cu and Zn) Complexes of 4-(1H-Imidazo[4,5-f][1,10]Phenanthroline-2-yl)-N,Ndimethylbenzene Amine

Biography:
Dr. Md Abdus Subhan received his PhD from Osaka University, Japan in March 2003. In 2004 he had been a Venture Business Laboratory (VBL) postdoctoral fellow at Materials and Life science, Faculty of Engineering, Osaka University for a year. Then he joined Shah Jalal University of Science and Technology, Sylhet, Bangladesh as faculty. In 2010 he visited Osaka Kyoiku University, Japan as visiting researcher to study the PL properties of mixed metal nano composite oxides. He had been National Research Foundation (NRF) postdoctoral fellow at Andong National University, South Korea for a year (2010–2011), where he studied novel macrocyclic metal complexes. He had been BK21 postdoctoral fellow at Seoul National University, South Korea for six months (2012–2013). At present he is a Professor and Head of Department of Chemistry at Shah Jalal University of Science and Technology, Sylhet, Bangladesh. His major research field includes novel metal complexes of materials, medicinal and biological importance as well as structure, spectroscopy, catalysis and photocatalysis, anti-microbial activity of multi-metal nanocomposite oxides.
Abstract:
Novel ligand, 4-(1H-Imidazo [4,5-f] [1,10] Phenanthroline-2-yl)-N,Ndimethyl benzene amine(L) (Scheme) was synthesized from 1,10-Phenantroline. Nano transition metal (Co, Fe, Ni, Cu and Zn) complexes of this ligand have been synthesized. Another metal complex of cobalt with (E)-2-styryl-1H-Imidazo[4,5-f][1,10]phenanthroline(L¢) ligand has also been synthesized and characterized. The ligand and the metal complexes were characterized by UV-Visible, FTIR, AFM and Mass spectrometry. AFM study (scheme) showed that the average roughness of the [Co(L)2(Ac)2], [Fe(L)2(Ac)2], [Ni(L)2(Ac)2], [Cu(L)2(Ac)2], [Zn(L)2(Ac)2] and [Co(L¢)2(Ac)2] are 14, 10.9, 5.9, 73, 4.42 and 101.42 respectively. The particle size of the metal complexes [Co(L)2(Ac)2], [Fe(L)2(Ac)2], [Ni(L)2(Ac)2], [Cu(L)2(Ac)2], [Zn(L)2(Ac)2] and [Co(L¢)2(Ac)2] observed are 73.7, 184.6, 145, 222.45, 62 nm and 1.33 μm, respectively,
DNA binding study of the synthesized metal complexes showed that the nano metal complexes are strong DNA binding and cleaving materials. The study of micellar behavior of SDS in presence of metal complexes in aqueous solutions was carried out by measuring the absorbance of 1-(2-pyridylazo)-2- naphthol (PAN) at 470 nm. The complexes [Co(L)2(Ac)2], [Fe(L)2(Ac)2], [Ni(L)2(Ac)2], [Cu(L)2(Ac)2], [Zn(L)2(Ac)2] and [Co(L¢)2(Ac)2] were found to decrease the CMC value of SDS. Negative values for of SDS are observed for the metal complexes. Negative values of indicated that the process of micellization is thermodynamically favorable. In this presentation we will also discuss our recent work on trimetallic nanocomposite oxide particles and their medicinal applications.
Shahd Abuhelal
King’s College London, UK
Title: The Combination of pH-Responsive Peptide and Cationic Liposomes Can Improve siRNA Transfection Efficiency in Cancer Cells

Biography:
Shahd obtained a BSc in Pharmacology with first class honor from Al-Quds University, Jerusalem in 2009. She then worked as a Rheumatology biopharmaceuticals product specialist at Roche Pharmaceuticals in Palestine and took part in various healthcare projects. She completed an MSc in Biopharmaceuticals with a distinction at King's College London, United Kingdom where she is currently doing her PhD research. Shahd is investigating the use of nanotechnology to develop a smart delivery systems to help overcoming cell barriers for siRNA to aid the treatment of cancer.
Abstract:
The presence of small interfering RNA (siRNA) in a cell leads to silencing of protein expression, offering the potential of a powerful therapeutic option for treatment of many conventionally intractable diseases. Due to the negative charge and sensitivity against RNase, siRNA has a short half-life in the blood and struggles to penetrate within the target cells. Successful development of siRNA therapies depends on the ability for it to be efficiently delivered inside the target cells efficiently while avoiding enzymatic degradation and aggregation. Methodology: We present the design and optimization of a cationic liposomal/pH responsive cationic peptide based nano-carrier for siRNA delivery. The major used components in the formulation are; DODAG (1 N′,N′-dioctadecyl-N-4,8-diaza-10aminodecanoylglycine amide), a cationic lipid which was designed to be a structural chimera involving the N(1)-cholesteryloxycarbonyl-3-7-diazanonane-1,9-diamine (CDAN) polar head group and the dialkylglycine amide moiety of dioctadecylamido glycylspermine (DOGS) to improve positively charged lipid interaction with siRNA, and LAH4-L1 is a 26-amino acid, histidine rich helical peptide. It is a membrane-active peptide that displays increased affinity toward anionic lipids and possess DNA delivery capabilities. Liposomes (20% DODAG, DOPC/ cholesterol/DSPE-PEG2000)/siRNA, LAH4-L1/siRNA (Figure1) and their combination with siRNA (Figure 2) have been investigated as delivery systems. Their physicochemical characteristics such as size, zeta potential, siRNA retention, release behaviour and aggregation behaviour of formulations were tested. In vitro cell uptake and luciferase knock-down studies were also tested in MDA-MB231 and A549 cells. Complexes were tested in vivo for bio-distribution. Findings: The hydrodynamic diameter and zeta potential of the particles was characterized. Size was adjusted to be around 130nm and zeta potential was slightly positive. All complexes associated with siRNA have more than 80% complexation efficiency. Lipoplexes showed better encapsulation but were less stable as leakage was observed after co-incubation in serum. Improved cell uptake was seen with ternary complex in comparison with liposomal and Peptide siRNA complexes (siRNA cells uptake was increased from 1.4% to 32.9% in some cases). In vitro studies suggest improvement in gene silencing with ternary complex even when compared with generic positive control. in vivo studies are ongoing to understand the pharmacokinetic behaviour of the particles. Conclusion & Significance: A stable lipid/peptide ternary complex for siRNA delivery with defined physicochemical properties was designed and evaluated and shown to be a promising delivery system for siRNA for both in vitro and in vivo applications. Current and future work is focused, in vitro, on the improvement of cell targeting and in vivo on studying the PK/PD properties of the new system.
L. Nandhakumar
Sri Indu Institute of Pharmacy, INDIA
Title: Role of various flavonoids: Hypotheses on novel Nanotechnology approach to treat diabetes

Biography:
L. Nandhakumar is a Professor & Head, Sri Indu Institute of Pharmacy, Ibrahimpatnam, Hyderabad- 10, and INDIA. Researcher in studying the plant components for the treatment of diabetes in novel Nanotechnology, Also focusing on the antioxidant studies in treating diabetes
Abstract:
Basically flavonoids are naturally occurring phenolic compounds that are distributed in plants. They contain wide range of biological activity and lot of research has been carried out on their potential role in treating diabetes and other diseases. Most importantly the flavonoids and their related natural compounds are known to encompass antidiabetic potential, demonstrated in various animal models. Such beneficial flavonoids are less utilized on account of its deprived solubility, decreased bioavailability; first pass metabolism and intestinal degradation. However, flavonoids are capable of improving, stabilizing and long sustaining the insulin secretion, human islets and pancreatic cell respectively. Methodology & Theoretical orientation: In this article we propose, remarkable antidiabetic activity of flavonoids as well as few approaches on nanoparticulate systems in diabetes induced animal models. The proposed nanoparticulate system of flavonoids is projected to improve the solubility, bioavailability, by passing the first pass metabolism and decreasing susceptibility to intestinal environment as compared to pure flavonoid isolates. Conclusion and Significance: Further, this hypothesis exemplifies to enhance the efficacy of flavonoids in a novel way of antidiabetic treatment.
Khalid AbdelRahim
King Saud University, Saudi Arabia
Title: Extracellular biosynthesis of silver nanoparticles using Rhizopus stonililifer

Biography:
Khalid AbdelRahim interested in antibiotics resistance problems. He is trying to help in overcoming this problem by awareness towards antibiotics usage, new drug by using nanotechnology
Abstract:
Synthesis of silver nanoparticles (AgNPs) has become a necessary field of applied science. Biological method for synthesis of AgNPs by Rizobus stonilifer aqueous mycellical extract was used. The AgNPs were identified by UV–visible spectrometry, X-ray diffraction (XRD), Transmission electron microscopy (TEM) and Fourier transform Infrared spectrometry (FT-IR). Presence of surface plasmon band around 420 nm indicates AgNPs formation . The characteristic of the of AgNPs within the face-centered cubic (fcc) structure are indicated by the peaks of the X-ray diffraction (XRD) pattern corresponding to (111), (200) and (220) planes. Spherical, mono-dispersed and stable AgNPs with diameter around 9.47 nm were prepared and affirmed by high- resolution transmission electron microscopy (HR-TEM). Fourier Transform Infrared (FTIR) shows peaks at 1426 and 1684 cm-1 that affirm presence of coat covering protein the AgNPs which is known as capping proteins. Parameters optimization showed smallest size of AgNPs ( 2.86 ± 0.3 nm ) was obtained with 10−2 M AgNO3 at 40 º C. Present study provides the proof that the molecules within aqueous mycellial extract of R. stonilifer facilitate synthesis of AgNPs and highlight on value-added from R. stonilifer for cost effective. Also, eco-frindely medical and nanotechnology-based industries could also be provided. Size of prepared AgNPs could be controlled by temperature and AgNO3 concentration. Further studies are required to study effect of more parameters on size and morphology of AgNPs as this will help in control of large scale production of biogenic AgNPs.
Neeraj Mishra
I.S.F. College of Pharmacy, India
Title: Surface modified microparticulate carriers of Embelin for their beneficial Pharmacological potential in ulcerative colitis

Biography:
Neeraj Mishra is working as Professor in Department of Pharmaceutics at ISF College of Pharmacy, Moga (Punjab) since 2012. He has completed his B. Pharm (2000), M. Pharm (2003) and Ph.D.(2011) in Pharmaceutical Sciences from Department of Pharmaceutical Sciences, Dr. H.S. Gour Central University, Sagar (M.P.). He was qualified in National Level Test GATE conducted by IIT, Kanpur in 2001. He is having around fifteen years teaching experience at post graduate and under graduate level. He is also having four years of research experience in Department of Pharmaceutical Sciences, Dr. H.S. Gour Central University, Sagar (M.P.). (2006-2010). He is also having one year of industry experience as production chemist in Symbiotec Pvt. Ltd., Indore (2000-2001). He was recipient of ICMR- SRF (New Delhi, India) (Grant: 45/02/2007-BMS/ PHA Dated 0672007H.S.Gour) during his Ph.D. tenure.
He is having 43 International and 12 National Publication typically in recent concept of novel drug delivery system, particularly in vaccine delivery and drug targeting. He is also written 2 book chapter in national and International publisher (Nova Science Publishers). He is having membership of the Indian Pharmaceutical Association (Life Time membership MP/IND/LM/0086) and Association of Pharmaceutical Teachers of India (APTI) life membership No. is PU/LM-379.
In Addition to this he is also acts as a reviewer of International repute journal (Journal of Microencapsulation (Informa Pharmaceutical Science). He has successfully organized one AICTE sponsored national seminar on “Emerging trends and applications of nanotechnology” on 11th June 2011 as Organizing secretary as organizing secretary in Swami Vivekanand College of Pharmacy, Indore (M.P.). He has also presented his research work in National and International conferences.
Abstract:
Present study was aimed to developed enteric coated microsphere of Embelin and their pharmacological potential was investigated in acetic acid induced ulcerative colitis. The optimized formulation of embelin loaded microspheres has shown significant sustained release of embelin. Further this formulation significantly reduced the ulcer activity score, oxidative stress and attenuates the inflammatory changes. Thus it may be concluded that embelin loaded enteric coated microparticles has shown delayed release capacity than plain Embelin and exerts colon ulcer protective effect in rats.
