
Sandeep Palvai
Indian Institute of Science Education and Research (IISER)-Pune, India
Title: Targeting Phosphatidylinositol-3-Kinase Signaling with Simultaneous DNA Damage in Cancer Cells by using Cholesterol based Chimeric Nanoparticle
Biography
Biography: Sandeep Palvai
Abstract
Phosphatidylinositol-3-kinase (PI3K) signaling has been a major target as it is found to be mutated and over expressed in many types of cancers. PI3K inhibition alone by small molecules failed to offer effective therapy since drug resistance has been a major problem. Recent studies reveal that inhibiting singling pathways with simultaneous DNA damage is effective to combat cancer. However, targeting PI3K signaling with small molecules in combination with DNA damaging drugs would be challenging as it leads to severe side effects due to nonspecific interactions in cancer patients. To this end, we have developed a biocompatible, biodegradable cholesterol-based chimeric nanoparticle (CNP), which can simultaneously load PI103, cisplatin and doxorubicin in a controlled ratiometric manner. Size, shape, and morphology of these CNPs were characterized by dynamic light scattering (DLS), ï¬eld-emission scanning electron microscopy (FESEM), atomic force microscopy (AFM), and transmission electron microscopy (TEM). CNPs demonstrated increased release of PI103, doxorubicin, and cisplatin through sustainable manner over 120 h at pH = 5.5 compared to neutral pH. The CNPs showed much improved in vitro cytotoxicity in HeLa,
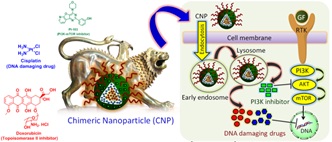
HL60, MCF7, and MDA-MB-231 cancer cells compared to a free drug cocktail at 24 and 48 h also induced apoptosis. Confocal laser scanning microscopy (CLSM) imaging revealed that these CNPs were internalized into cells through endocytosis in a time dependent mode over 6 h and retained inside for 48 h in HeLa, MDA-MB-231, and MCF7 cells. These CNPs exhibited their efficacy by damaging DNA and inhibiting Akt as a downstream modulator of PI3K signaling in HeLa cervical cancer cells. To conclude, these CNPs have the potential to open up new directions in next-generation nanomedicine by targeting multiple oncogenic signaling pathways with simultaneous induction of DNA damage for the augmented therapeutic outcome by reducing off-target toxicity and overcoming drug resistance.

