Day 2 :
Keynote Forum
Roger M. Leblanc
University of Miami, USA
Keynote: Development and Bio-applications of Nontoxic C-Dots
Time : TBA

Biography:
Roger M. Leblanc received a B.Sc. degree in Chemistry from Université Laval in 1964, followed by a Ph.D. in Physical Chemistry in 1968. Then, he obtained a postdoc position at the Royal Institution of Great Britain for two years before moving to the University of Québec, Trois-Rivières, Canada, where he spent 20+ years of studying photobiophysics. He moved his research to the University of Miami in 1994. Dr. Leblanc is Professor and Chair of Chemistry Department at University of Miami. And his research interests are centered on biophotophysics, spectroscopy and surface chemistry and he has published more than 500 research articles related to these topics and has guided more than 100 Ph.D. and M.Sc.
Abstract:
Carbon dots (C-Dots) have recently attracted enormous attention due to their unique properties. In this talk, the synthesis, characterization and bio-applications of a new type of nontoxic, water-soluble C-Dots will be presented. A major medical challenge one faces to treat central nervous system (CNS) related diseases is to cross the tight junctions between endothelial cells, which are known as blood–brain barrier (BBB). Recently, our in vivo experimental observations suggested that the transferrin conjugated C-Dots could enter the CNS of Zebrafish while C-Dots alone could not. Thanks to the abundant presence of carboxylic acids on the surface, C-Dots are easily conjugated with transferrin and anticancer drug doxorubicin.

The system was then applied as a drug delivery system for the delivery of doxorubicin into cancerous cells. Our in vitro study showed greater uptake of the conjugates compared to free doxorubicin, the conjugates at 10 nM was significantly more cytotoxic than doxorubicin alone, reducing viability by 14~45 %, across multiple pediatric brain tumor cell lines. Accidents, disease and aging compromise the structural and physiological functions of bones, and in vivo bone imaging test is critical to identify, detect and diagnose bone related development and dysfunctions. Here we show that C-Dots with low quantum yield (“dark”) bind to calcified bone structures of live Zebrafish larvae with high affinity and selectivity. Binding resulted in a strong enhancement of luminescence that was not observed in other tissues, including non-calcified endochondral elements. Retention of C-dots by bones was very stable, long lasting, and with no detectable toxicity. These observations support a novel and revolutionary use of C-Dots as highly specific bioagents for bone imaging and diagnosis, and as a potential bone-specific drug delivery carrier.
Keynote Forum
N. Benkirane jessel
INSERM, France
Keynote: Living Implant Fortified with active therapeutics and Well organized Stem cells spheroids for Regenerative NanoMedicine
Time : TBA

Biography:
Nadia Benkirane is Research director and head of the “ Osteoarticular and Dental regenerative Nanomedicine” laboratory, at INSERM (French National Institute for Health and Medical Research), UMR 1109, Strasbourg, France. She was leader of “Active Biomaterials and Tissue Engineering” team INSERM 977. She received her Ph.D. from University Louis Pasteur, ULP, Strasbourg, France for the work on Development of pseudopeptides as synthetic vaccines. Dr. Jessel (Benkirane) then held a postdoctoral position in collaboration with the Institut Pasteur, Paris, France, working on Immunotherapy HIV, and another postdoctoral position on the application of modified peptides as vaccines against FMDV (Plum Island Animal Disease Center, ARS, USDA, Greenport, NY 11944-0848, USA). She joined the INSERM U595 in 2002 as a post-doc, and received the diploma to direct the research (HDR) in 2004. Dr. Jessel got the permanent position (CR1) in the INSERM 595 laboratory in 2004 and Research Director (DR2) position in the INSERM 977 and head of “active Biomaterials and Tissue Engineering team from 2009 until 2012). Currently Research Director (DR1) in the INSERM UMR 1109 (Osteoarticular and Dental Regenerative Nanomedicine" and heads the team. Dr. Jessel possesses expertise in diverse fields of molecular and cellular biology, immunochemistry, tissue engineering and biomedical engineering. In the last 10 years, she focused her research on the bio-functionalization of multilayered polyelectrolyte architectures with emphasis on the use of these architectures to induce specific cellular responses and gain control over cell proliferation and differentiation. Dr. Benkirane-Jessel have 138 publications (h index: 36) with peer-reviewed publications in high impact factor journals (Proc. Nat. Acad. Sci. USA; Adv. Mater.; Adv. Funct. Mater.; Small; Nanoletters, Biomaterials, ACS Nano), 5 chapters reviews and 5 international patents, she is a regular referee for a number of scientific journals Nature nanotechnology, Nature Materials, ACS nano, Biomaterials, Nanoletters… She is under the contract (Interface INSERM/Clinic 2008-2013) and she got also “Prime d’Excellence Scientifique” from the INSERM, 2010-2014 and the PEDR from the INSERM on 2016 for 4 years.
Abstract:
Recently, We have reported an active nanostructured collagen implant reinforced with human stem cells for bone regeneration. In our group, we have reported a "Smart Hybrid Materials Equipped with Nanoreservoirs of Therapeutics and stem cells spheroids ". This unique nanotechnology strategy is used to entrap, protect, and stabilize therapeutic agents into polymer coatings acting as nanoreservoirs enrobing nanofibers of implantable membranes. Upon contact with cells, therapeutic agents become available through enzymatic degradation of the nanoreservoirs. As cells grow, divide, and infiltrate deeper into the porous membrane, they trigger slow and progressive release of therapeutic agents that, in turn, stimulate further cell proliferation. This constitutes the first instance of a smart living nanostructured hybrid membrane for regenerative medicine. The cell contact-dependent bioerodable nanoreservoirs described here will permit sustained release of drugs, genes, growth factors, etc., opening a general route to the design of sophisticated cell-therapy implants capable of robust and durable regeneration of a broad variety of tissues.
Clinical trial: phase 1 Horizon 2020, (FR, UK, SP, SW) submitted
Feasibility and safety assessment of a therapeutic implant based on a bioactive collagen membrane and autologous mesenchymal stem cells derived from bone marrow for the treatment of femoral cartilage isolated lesions
Keynote Forum
Li Li
University of Queensland, Australia
Keynote: Hierarchical layered double hydroxide nanocomposites for drug and siRNA delivery

Biography:
Dr Li Li is an Advance Queensland Research Fellow (Mid) at Australian Institute for Bioengineering and Nanotechnology, University of Queensland. She is a materials scientist with extensive experience in nanoparticle synthesis and applications in targeted drug delivery and vaccination. She has developed several functional NPs platforms including layered double hydroxides (LDHs), silica NPs and nanoemulsions, and applied these NPs to efficiently deliver anti-cancer drugs and siRNA for cancer treatment. She has employed LDH-based nanoparticles to co-deliver drugs and gene to improve drug efficiency in cancer treatment. This new strategy provides a promising approach for advance cancer therapy. She established the close relationships with the national and international experts, published high quality research papers in Adv Mater, Biomaterials, Nano Letters, Nano research, Adv Funct Mater.
Abstract:
Chemotherapy is one of most common cancer treatments in clinics. In most cases, the clinical responses show that the efficacy of chemotherapy is limited by the development of multidrug resistance (MDR) in cancer cells during a long period of treatment. Target-specific delivery and sustained release of anticancer agents and siRNA has attracted considerable research interest in cancer chemotherapy. It is clear that the single treatment by either anticancer drug or siRNA delivered by nanocarriers can only achieve limited success in overcoming the MDR of cancer cells. Thus, the development of an effective strategy to overcome the multidrug resistance in chemotherapy remains a major challenge in the treatment of cancers, where co-delivery of anticancer drugs and siRNA would be a promising strategy.
Recently, hierarchical nanocomposites have attracted great interests in bioapplications such as drug delivery, biomedical imaging, biochemical sensing and biocatalysts owing to their structure features and unique properties.1 In our group, we have developed hierarchical SiO2@MgAl-layered double hydroxide nanocomposites (SiO2@MgAl-LDH) with various functional groups (-NH2, -SH, -PEG) via nanodot-coating strategy. These nanocomposites have showed enhanced siRNA and drug delivery to cancer cells. The functional SiO2@MgAl-LDH nanocomposites retained the layered structure and plate-like morphology as MgAl-LDH NPs. Moreover, functional SiO2@MgAl-LDH showed good dispersion in aqueous solution and cell culture medium. The in vitro tests have demonstrated anticancer drugs or siRNA delivered by functional SiO2@MgAl-LDH apparently inhibited the cancer cell growth.2. 3
- Novel Drug Delivery Systems | Nanomaterials for drug delivery
Session Introduction
Lucas B Naves
University of Minho, Portugal
Title: Polycaprolactone blended with Linear and Branched Polyethynimines scaffolds for skin regeneration treatment – In vitro study
Biography:
Lucas Bernardes Naves is currently persuing his Ph.D at the University of Minho, Portugal at the Center for Science and Textile Tecnology. Under supervision of Prof. Luis Almeida He is also doing his research at the National University of Singapore (NUS), where he is working at the Center for Nanofiber and Nanotechnology under the supervison of Prof. Seeram Ramakrishna. His research leads to the development of biomaterials for skin tissue engineering, focusing on the treatment of less invasive approach for melanoma skin cancer.
Abstract:
Skin regeneration is a huge issue over the last few decades. As a result of burns, trauma, diabetes and several other diseases, skin grafts are needed, aiming the regeneration of the injured body site. In this paper, we present a new alternative approach, a comparison of linear and branched Polyethylenimine. This research presents the viability and biocompatibility of LPEI and BPEI loaded with polycaprolactone (PCL) scaffolds.
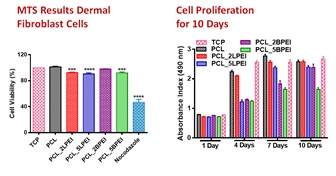
Figure: MTT assay showing the biocompatibility of all the scaffolds with Human Dermal Fibrblast cells.
SEM images show that the scaffolds developed sized between 74± 419 nm. Contact angle assay demonstrated high hydrophobicity for all mats, which could be overcome by surface modification, plasma treatment, helping the hydrophilicity of the mats, providing excellent of the cells adhesion to the surface of the scaffolds. We demonstrate the biocompatibility of the scaffolds developed by electrospinning techniques, followed by in vitro tests with Human Dermal Fibroblast (HDF), by using MTT assay to determine the biocompatibility with the cells, and the Sirius red collagen to determine the relies profile after six days of cells incubation. The results have shown that all the scaffolds developed to have good cells adhesion, cell biocompatibility for HDF, good collagen release profile for all mats, increased release on the day 10. This primary in vitro study suggest that the mats developed may increase in the skin regeneration process, therefore can be an emerging technology for skin regeneration.
Shahd Abuhelal
Institute of Pharmaceutical Science, King’s College London, United Kingdom
Title: The Combination of pH-Responsive Peptide and Cationic Liposomes Can Improve siRNA Transfection Efficiency in Cancer Cells

Biography:
Shahd obtained a BSc in Pharmacology with first class honor from Al-Quds UniversityJerusalem in 2009. She then worked as a Rheumatology biopharmaceuticals product specialist at Roche Pharmaceuticals in Palestine and took part in various healthcare projects. She completed an MSc in Biopharmaceuticals with a distinction at King's College London, United Kingdom where she is projects. She completed an MSc in Biopharmaceuticals with a distinction at King's College London, United Kingdom where she is overcoming cell barriers for siRNA to aid the treatment of cancer.
Abstract:
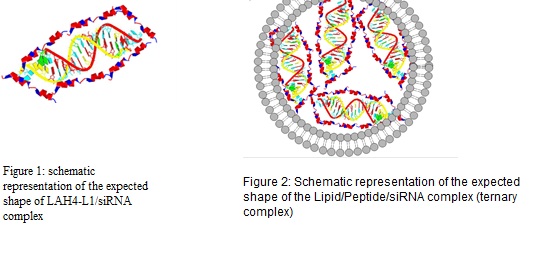
The presence of small interfering RNA (siRNA) in a cell leads to silencing of protein expression, offering the potential of a powerful therapeutic option for treatment of many conventionally intractable diseases. Due to the negative charge and sensitivity against RNase,siRNA has a short half-life in the blood and struggles to penetrate within the target cells. Successful development of siRNA therapies depends on the ability for it to be efficiently delivered inside the target cells efficiently while avoiding enzymatic degradation and aggregation. Methodology: We present the design and optimization of a cationic liposomal/pH responsive cationic peptide based nano-carrier for siRNA delivery. The major used components in the formulation are; DODAG (1 N′,N′-dioctadecyl-N-4,8-diaza-10aminodecanoylglycine amide), a cationic lipid which was designed to be a structural chimera involving the N(1)-cholesteryloxycarbonyl-3-7-diazanonane-1,9-diamine (CDAN) polar head group and the dialkylglycine amide moiety of dioctadecylamido glycylspermine (DOGS) to improve positively charged lipid interaction with siRNA, and LAH4-L1 is a 26-amino acid, histidine rich helical peptide. It is a membrane-active peptide that displays increased affinity toward anionic lipids and possess DNA delivery capabilities. Liposomes (20% DODAG, DOPC/ cholesterol/DSPE-PEG2000)/siRNA, LAH4-L1/siRNA (Figure1) and their combination with siRNA (Figure 2) have been investigated as delivery systems. Their physicochemical characteristics such as size, zeta potential, siRNA retention, release behaviour and aggregation behaviour of formulations were tested. In vitro cell uptake and luciferase knock-down studies were also tested in MDA-MB231 and A549 cells. Complexes were tested in vivo for bio-distribution. Findings: The hydrodynamic diameter and zeta potential of the particles was characterized. Size was adjusted to be around 130nm and zeta potential was slightly positive. All complexes associated with siRNA have more than 80% complexation efficiency. Lipoplexes showed better encapsulation but were less stable as leakage was observed after co-incubation in serum. Improved cell uptake was seen with ternary complex in comparison with liposomal and Peptide siRNA complexes (siRNA cells uptake was increased from 1.4% to 32.9% in some cases). In vitro studies suggest improvement in gene silencing with ternary complex even when compared with generic positive control. in vivo studies are ongoing to understand the pharmacokinetic behaviour of the particles. Conclusion & Significance: A stable lipid/peptide ternary complex for siRNA delivery with defined physicochemical properties was designed and evaluated and shown to be a promising delivery system for siRNA for both in vitro and in vivo applications. Current and future work is focused, in vitro, on the improvement of cell targeting and in vivo on studying the PK/PD properties of the new system.
Mansoureh Nazari Vishkaeia
Universiti Sains Malaysia, Malaysia
Title: Anti-angiogenic Efficacy Enhancement in Nano Phospholipid complex of Orthosiphon Stamineus Ethanolic Extract

Biography:
Mansoureh is PhD research student at School of Pharmaceutical Sciences, Universiti Sains Malaysia. She has expertise in the development of nano formulation of orthosiphon stamineus ethanolic extract, a poorly water soluble extract with potent anticancer activity. She has used phosphatydilcholine as career which is an essential part of the cell membranes to develop this unique formulation. This agent not only acts as a carrier of water insoluble extract but also nourishes the lipid membranes of the cells.
Her formulation has the potential to be manufactured in large scale and can be used as a carrier for other therapeutic drugs.
Abstract:
Orthosiphon stamineus (O. stamineus) is a therapeutic herb with significant pharmacological properties. High dose of O.S extract had demonstrated some potent antiangiogenic and antitumor activities. It had weak competency to cross the biological membranes because of consisting of large size of rings in its active compounds and highly water solubility. This study aims to develop and characterize new O.S-phospholipid complex to reveal more antiangiogenic efficacy by incorporating with phosphatidylcholine to form new cell like structure which is amphipathic in nature.
O.S-Phospholipid complex was prepared by the modified film method, and characterized for its physico-chemical properties. Ex-vivo and in-vitro antiangiogenic studies demonstrated efficacy improvement after formulation.
Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), particle size and HPLC analysis confirm the formation of phospholipid complex. Phospholipid complex of O.S solubility increased in active compounds with 89.21±45.44% entrapment. Ex-vivo study showed 50% improvement in antiangiogenic activity at high dose. Inter (n=3) and intra (n=5) lab experiment were used for confirmation.
Thus, this study demonstrated new nano O.S-Phospholipid complex as a promising method to enhance antiangiogenic activity. Moreover, this method can be used as a sustained delivery system for hydrophilic compounds with poor lipid-solubility and low oral bioavailability which improves pharmacological properties.
Zheng Ruan
University of Science and Technology of China, China
Title: pH-sensitive amphiphilic polypeptide prodrug for NIR imaging-guided combined photodynamic therapy and chemotherapy
Biography:
Zheng Ruan, University of Science and Technology of China, China
Abstract:
Photodynamic therapy (PDT) is a promising clinical modality for the treatment of tumours and non-malignant nidus. A 4,4-difluoro-4-bora-3a,4a-diaza-sindacene (BODIPY) core based photosensitizer (PS) has many of the ideal characteristics of a PDT agent, such as a high extinction coefficients, resistance to photobleaching, and high ratios of light–dark toxicity which could also be recognized as dye for bioimaging. However, the BODIPY mentioned above cannot be dispersed in aqueous solution since it is hydrophobic, and therefore needs a BODIPY-carrier so that the PDT agent can be delivered and then released in the areas of tumors to kill cells. And few researchers have combined chemotherapy using DOX with a photosensitizer which shows great lethality to cancer cells like HepG2 and eminent bioimaging ability. Owing to the significant acidic microenvironments of tumour tissues and low pH (∼5.0) inside cancer cells, we have innovatively tried to synthesize hydrazone based pH-sensitive peptide within PEG shells and entrapped by the novel NIR-BODIPY photosensitizer using DOX for anticancer curing (Figure 1a). The integration of chemotherapy and PDT has meet the rising necessity of combination for clinical diagnosis. All the polymeric micelles are of suitable size for the EPR effect and can be easily disassembled in an acidic microenvironment to release the DOX or BODIPY for cancer treatment (Figure 1b). The polypeptide itself shows great biocompatibility to cells, while severe damage to HepG2 cancer cells was caused by micelles with BODIPY and DOX which have also shown well NIR-imaging ability (Figure 1c). All those advantages above mean that dual-agent pH-sensitive polypeptides may be promisingly applied in future medical cures in a combination of PDT and chemotherapy.
Akash Arya
Biophotonics Laboratory, Indian Institute of Technology, India
Title: High yield synthesis of surfactant USA) -free gold nanostars for biosensing, photothermal therapy and drug delivery applications

Biography:
Akash Arya obtained his B.E. Degree in Electronics and communication engineering, and M.Tech (Nanoscience & Technology) degree from Pondicherry University. Currently, he is a senior Research scholar at Biophotonics Laboratory at Indian Institute of Technology (IIT) Patna. His current Research interests are (i) synthesizing different kinds of nanoplasmonic structures using different wet chemistry methods for biosensing and drug delivery applications and (ii) fabricating novel nanoplasmonic-photonic hybrid biosensors and demonstrating the detection and sizing of single protein molecules using the developed biosensors.
Abstract:
Development of drug delivery system (DDS) plays a vital role in the field of biomedicine and healthcare, where maximum therapeutic effect and minimum undesirable side effect is the key of success for ideal DDS. Various methods of DDS using nanoparticles, microspheres and hydrogels are commercially available to treat diverse diseases ranging from cancer to fungal infection and to muscular degeneration. Among nanoparticles, gold nanoparticles demonstrate special advantages in this field due to their unique properties, small size and high surface area-tovolume ratio. These particles have been widely used in various biomedical applications and drug delivery systems due to their inert nature, stability, high dispersity, non-cytotoxicity and biocompatibility.

On the other hand, Researchers have been using the gold nanoparticles for fabricating nanoplasmonic-whispering gallery mode hybrid microresonators [2,3] and surface enhanced Raman scattering (SERS) based biosensors [4] for the real-time detection of single protein molecules at their natural state. This detection would be extremely useful for predicting dangerous diseases such as cancers at very early stage. A few years back, gold nanostars are found to be efficient for biosensing, photothermal therapy and drug delivery applications. A few wet chemistry methods existing in the literature for synthesizing the nanostars in one step. However, the yield of the nanostars are found be very low (40-45%). In contrast to this, recently we have successfully synthesized the surfactant-free gold nanostars in one-step, using a novel wet chemistry method [5]. In contrast to the existing reports, these nanostructures have longer and sharper spikes in all directions. From scanning electron microscopic images, the estimated yield of the nanostars was more than 95%. Details of synthesis and characterization of star shaped gold nanostructures, and usefulness of these nanostars in biosensing, photothermal therapy and drug delivery applications would be explained in detail with the help of numerical simulations based on finite element method at Nanodelivery 2017.
Julissa Gonzalez Villegas
University of Puerto, Rico
Title: Surface derivatization of zirconium phosphate nanoplatelets: Potential nanocarrier for doxorubicin
Biography:
Julissa González was born in San Juan, Puerto Rico. After participated in national and international scientific fairs at secondary level she decided to study Chemistry in University of Puerto Rico-Río Piedras campus where she obtained a BS degree. Currently she is a Ph.D. candidate in Inorganic Chemistry area and work with surface modification of zirconium phosphate nanoparticles for drug delivery system applications. In addition, she is member of the Caribbean Brigade of the Solar Army during the last five years as part of the Center for Chemical Innovation in Solar Fuels (CCI Solar) an NSF Center for Innovation in Solar Fuels.
Abstract:
Surface modification of doxorubicin anticancer drug (DOX) intercalated zirconium phosphate (ZrP) nanoparticles (DOX@ZrP) is proposed to improve the potential of this drug delivery system for cancer therapy. The surface of DOX@ZrP nanoparticles was modified with an amorphous layer of Zr(IV) followed by modification with monomethyl-polyethylene glycol-monophosphate (m-PEG-PO3) to increase the DOX@ZrP biocompatibility. 31P{1H}MAS NMR data shows a new peak at -26 ppm corresponding to the PO43- groups coordinated with Zr(IV) on the surface. m-PEG-PO3/Zr(IV)/DOX@ZrP spectra shows no additional resonance centered at d of -22.6 ppm generated by proton-phosphorous cross polarization indicating no partial PEG intercalation in the interlaminar space. Simulated body fluid (SBF) was used to determine the in vitro release of DOX from DOX@ZrP, Zr(IV)/DOX@ZrP, and m-PEG-PO3/ Zr(IV)/DOX@ZrP. MTS cell viability assay reveal that m-PEG-PO3/ Zr(IV)/DOX@ZrP exhibited a 20% increase in the toxicity comparing with free DOX when PC3 cells are exposed for 48 h. m-PEG-PO3 polymer coating of DOX@ZrP nanoparticles promise to have a strong impact on the targeting, distribution and degradation of the nanoparticles under physiological environment that should result in a more efficient chemotherapy agent than free doxorubicin.
Biography:
Mehran Ghiaci is a professor of chemistry at Isfahan University of Technology (Iran). He received his BS (1973) and MS (1974) in chemistry from Pars College (Iran). He received his PhD degree (1980) in physical organic chemistry from University of California at Los Angeles (USA). His current research interests include physical organic chemistry, heterogeneous catalysts, organic synthesis, surface chemistry, chemical sensing and drug delivery.
Abstract:
Our main goal in presenting this methodology was to modify the conventional systemic delivery of drugs because such procedures may cause toxicity; for example, polymeric coatings may present some disadvantages such as limited chemical stability, local inflammatory reactions and so on. As a result, we thought that it could be interesting to embed bioactive compounds and biomolecules within inorganic coating such as TiO2, ZrO2, and SiO2. This type of coating increases drug passage time through its small and long pores forward intended fluid (whether in vitro or in vivo) and eliminates different stimuli such as (temperature, pH, ultrasonic irradiation,...) to remove the coating on the surface of drug carrier system. This could be very effective economically and time spent. Moreover, if such inorganic coatings have nanostructure properties, they improve cellular adhesion, enhances osteoblast proliferation, and increase biomineralization. In this talk, emphasis is placed on presenting the technique, and would like to explore it as a new methodology in drug delivery.
Darya Tsvirkun
Institute of Drug Research,The Hebrew University of Jerusalem, Israel
Title: Size-Dependent Targeted GNPs as CT Contrast Agents for Molecular Imaging of Cancer

Biography:
Darya Tsvirkun began her career oriented towards the development and evaluation of PET 11C-tracers for imaging and quantification of myocardial perfusion, receiving the Dean's Excellence Award. Her contemporary research focuses on development and synthesis of new libraries of activity based probes (ABPs) labeled with various CT contrast agents for cysteine proteases for cancer imaging. This project will significantly advance the molecular imaging field that is rapidly developing world-wide by creating dramatically enhanced imaging reagents. Having tools that can allow non-invasive imaging of protease activity in vivo is a powerful research tool. The use of ABPs to investigate biological regulation is an exceptionally powerful method as it enables real time monitoring of enzymatic activity and localization in vivo. Most importantly, CT probes that identify cathepsin activity can be widely applied to image human tumor location and grade, and can also be used to determine therapy efficacy.
Abstract:
In this study, we generated a new classes of cathepsin targeted probes based on different sizes of GNP (10, 30, and 100 nm) for functional CT imaging of cancer. ABPs are small molecules that have been engineered to covalently modify enzyme targets in an activity dependent manner. These novel probes enable detection of the elevated cathepsin activity within cancerous tissue using a CT instrument, thus creating a direct link between imaging signals and biological process.
X-ray CT instruments are among the most available, efficient and cost-effective imaging modalities in hospitals. The field of CT molecular imaging agents is emerging relying mainly on detection of gold and bismuth nanoparticles, iodine and gadolinium labeled compounds. However, the low sensitivity of CT scanners to contrast reagents in comparison to other imaging modalities makes this a challenging task.

We have generated chemical scaffolds of GNP-ABPs with combination of different protective layers of PEG studied in terms of length (3 or 5 kDa) and ratio (10, 50, and 100%). Efficiency of targeting moiety, based on different PEG coatings, was evaluated for tumor accumulation and enzyme inhibition effectiveness. Using a combination of analytical methods as TGA, DLS and ZETA, we estimated the average number of PEG units (~0.21 PEG/ nm2) on one particle, conducting the peptide quantity for each derivative. After chemical and biochemical evaluations we selected the most potent and stable probes to proceed to non-invasive imaging in cancer mice models. CT contrast from the tumor could be detected 5 hours post injection of targeted GNP probes. This specific signal increased over time and was significant at 24-72 hours post injection, compared to non-targeted particles. Contrast agent concentrations and sub-cellular localization within the tumor cells was detected using TEM. In conclusion, we found our GNP-ABPs as a promising new tools for functional imaging of specific protease activity in-vivo by CT instrument.
Yue Hui
The University of Queensland, Australia
Title: Controlling the Stiffness of Biomimetic Silica Nanocapsules and Its Impact of Cellular Uptake

Biography:
Yue Hui is currently a PhD candidate from the Australian Institute for Bioengineering and Nanotechnology, the University of Queensland. He has an academic background of materials, chemistry and nanotechnology. Yue aims to develop a nanocarrier for drug delivery application based on a biomimetic dual-templating platform technology developed at The University of Queensland. He is now investigating the effect of nanocapsules’ stiffness on their biological performance including cellular uptake and biodistribution.
Abstract:
Over the past decades, advances in nanotechnology have led to the emergence of myriad nanomaterials that are promising for biomedical applications includingn bio-imaging and drug delivery. To better understand and predict the biological performance of these materials, effects of their physicochemical properties (e.g., size, charge and surface chemistry) have been extensively explored, providing valuable rules for the design of next-generation nanomaterials. Another equally important yet often overlooked character, the mechanical property of nanomaterials (e.g., deformability and stiffness), has recently been recognized to influence and even control their biological fates including vascular circulation and cellular uptake, while a better understanding upon this is still lacking. In this study, we synthesized oil-filled silica nanocapsules (SNCs) having variable stiffness and investigated the impact of their stiffness on both non-specific and ligand–receptor mediated cellular uptake. The prepared SNCs had a diameter of approximately 150 nm, a high-efficiency encapsulation (>90%) to the fluorescent dye DiI and good biocompatibility, with their stiffness ranging from highly deformable to rigid. Compared to deformable SNCs, rigid SNCs showed higher uptake in both macrophage and ligand–receptor mediated tumor cell internalization, while no significant difference was observed between their non-specific tumor cell uptake. These results indicate the existence of an optimal stiffness to balance non-specific macrophage clearance with receptor-mediated cellular internalization, which may guide the design of new nanomaterials that link chemistry, mechanics and biology for enhanced nanomedicine.
Seongjae Jo
Korea University, Korea
Title: Copper II ions detection via Localized Surface Plasmon Resonance
Biography:
Seongjae Jo studied control and measurement theory at the Department of Control and Instrumentation Engineering at Korea University. Among various subjects, He was interested in sensors and went to graduate school to study nano and biosensors. He is currently studying for the 5th semester of graduate school as a master and doctor Integrated course. He is studying sensing methods using optics such as absorbance, Raman scattering, and fluorescence, and he is also studying the synthesis of gold nanoparticles for optical applications. His study is supported by the National Research Foundation of Korea (NRF) under Grant no. NRF-2015R1A1A1A05027581 and Korea University Future Research Grant.
Abstract:
As the modern technology has developed, the problem to toxicity of nano-scale materials continues to rise, so it has emerged as an important research project to detect the toxic agents. In particular, according to a recent study, small amounts of copper ions increase the growth rate of the tumor, so it is important to detect a low concentration of copper ions.
A conventional method for detecting ions is a use of inductively coupled plasma (ICP) that is expensive and has a hassle things like preprocess of the samples and stabilization of the plasma which is necessary. However, the use of localized surface plasmon resonance (LSPR) is one of the techniques using the optics which can easily and quickly detect materials on the substrate in real time. The substrate and chelators, antibodies, aptamers or ligands are conjugated, can bind the desired materials like ions, proteins, even enzymes and genes.
In this study, we made nanoplasmonic substrates using gold nanorods and D-penicillamine (DPA). D-penicillamine (DPA) is easy to conjugate with gold due to thiol group. But it is a chelator of the copper, so the DPA conjugated to the substrate is separated from the substrate and bonded to the copper II ions. Copper ion could be detected up to 100 picomolar (pM) concentration by using the nanoplasmonic substrate. It shows a unique selectivity for copper II ions. And we were able to detect copper in human blood-like environment. Based on this study, not only copper II ions but also other ions can be detected by making the substrate that can be more quickly and easily monitored for ions.
Roberto Gonzalez Pizarro
University of Barcelona, Spain
Title: Fluorometholone –loaded nanospheres PLGA for the treatment of oculars disorders
Biography:
Roberto González completed his studies at University of Valparaiso (Chile) at the age of 25 with a thesis on the validation of the process of the fabrication of tablets in a pharmaceutical laboratory. After his studies he started to work at the National Agency of Medicines in Chile for 2 years as Inspector of the validation of the pharmaceutical processes. Subsequently he went to Barcelona to do a post-graduate course called Research, Development and Control of Drugs. Currently he is doing his PhD at University of Barcelona. His PhD project is about the development and characterization of nanostructured systems.
Abstract:
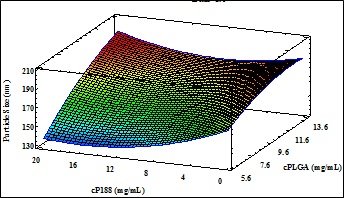
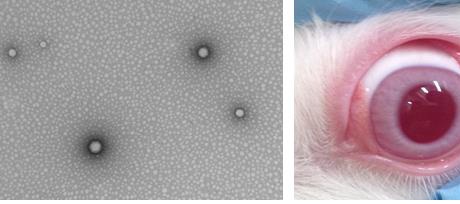
Fluorometholone (FMT) is a drug that is used in ophthalmology for inflammatory and allergic processes. However, commercials formulations have low residence time in the corneal area, obtaining no-effective therapeutic levels. The purpose of this study was to developed poly(lactic-co-glycolic-acid) (PLGA)-nanospheres (NSs) loaded with FMT in order to increase the ocular bioavailability of FMT. The NSs were prepared by the solvent displacement method using poloxamer 188 as surfactant and acetone as organic solvent. To optimize and to investigate the correlation between independents variables (such as the pH of aqueous phase, concentrations of poloxamer 188 and of the FMT) and the dependents variables (average particle size (Zav), polydispersity index (PI), zeta potential (ZP) and encapsulation efficiency (EE)) previously a factorial design was applied. At same time, the Zav of NSs was checked by transmission electronic microscopy (TEM). X-ray diffraction, infrared spectroscopy and differential scanning calorimetry were applied to evaluate interaction drug-polymer. Following this, the biopharmaceutical behavior (release in vitro) and the ex vivo ocular permeation of optimized formulation was studied using Franz cells. The interaction studies showed that there is no link formation between the drug and the other components of the nanostructured system. Also, the optimized formulation selected by factorial design showed a good stability which it was measured by Turbiscan®. The NSs evidenced a slow and prolonged profile release and a better bioavailability than commercials formulations. According to the above described, this developed nanostructured system of prolonged release could be used for the treatment of inflammatory and allergic ophthalmic disorders.
The authors would like to thank CONICYT (National Commission for Scientific and Technological Research) of Chile and the Spanish Ministry of Science and Innovation grant (MAT2014-59134R) for financial support.
Hamideh Salehi
University of Montpellier, France
Title: Dental pulp stem cells as anticancer drug delivery system

Biography:
Hamideh Salehi associate professor at university of Montpellier, France is working on the imaging of stem cells and cancer cells in different fields such as drug delivery, regenerative medicine and tissue imaging. She was previously postdoctoral fellow in biomedical engineering at Beckman Laser Institute at University of California Irvine, USA. She did research on lipid imaging in apoptotic cancer cells by coherent Raman spectroscopy CARS/SRS. She did her PhD in Nano biophysics in university of Montpellier 1, France. She has obtained two master degrees, the first in solid state physics- photonics crystal- and the second Mechanical engineering -shape memory polymers - in ENSAM, ParisTech.
Abstract:
Due to the lack of anticancer drug’s specificity, side effects of chemotherapy in cancer treatments are unavoidable and it has an impact on patient quality of life. Over the past 30 years, increasing efforts is done to optimize chemotherapy dosing to reduce drug toxicity while increasing its efficacy. A new study proves stem cells can act as a drug reservoir and they will release anticancer drug in its original form in nearby area of cancer cells. Stem cells, due to their capacity to uptake drug, can control the drug toxicity.Dental Pulp Stem Cells DPSCs are able to uptake Paclitaxel PTX and could release it in the culture medium gradually. The conditioned culture medium (culture medium plus released PTX from DPSC) is transferred to the breast cancer cells MCF7.Visualizing the drug uptake intracellular could provide us mechanism of action of the drug. Applying Confocal Raman Microscopy, anticancer drug uptake by MCF7 is imaged. Surprisingly MCF7 -without any direct contact with PTX- showed drug uptake. It proves the stem cells carry and deliver anticancer drug without its modification. It could be a revolution in chemotherapy to avoid the side effects and increase the drug efficacy.
Marcelle Silva de Abreu
University of Barcelona,Spain
Title: Encapsulation of agonist PPAR- γ in polymeric nanoparticles to treat ocular inflammatory processes

Biography:
Marcelle Silva de Abreu is currently a PhD student at the University of Barcelona, Faculty of Pharmacy and Food Sciences. She has a Master’s degree in Research, Development and Control of the Drugs. Her research is centered in the field of Nanoscience and Nanotechnology in the area of nanostructured drug delivery systems.
Abstract:
Peroxisome proliferator-activated receptor gamma (PPARγ) is a member of the nuclear receptor superfamily of ligand-dependent transcription factors. PPAR𛾠has been shown in numerous studies to affect the expression of proinflammatory cytokines. Pioglitazone (PGZ), a PPARγ agonist used to treat type 2 diabetes, has been reported to have responses in different inflammatory processes. The purpose of this study was the association of PGZ to poly (D,L-lactide-co-glycolide) poly(ethylene glycol) (PLGA-PEG) nanoparticles (NPs), for the treatment of ocular inflammatory disorders. NPs of PGZ were prepared by solvent displacement technique. Previously, a factorial design was carried out to determine the influence of independents variables studied. Physicochemical characterization,
biopharmaceutical behaviour and interaction drug-polymer studies were done. To evaluate ocular tolerance of the developed formulation, HET CAM and DRAIZE test were performed. In order to analyze the effectiveness of these systems, in vivo studies were executed in rabbits (male/n=6 per group), before and after induction of ocular inflammation by Sodium Arachidonate (SA). Results obtained demonstrated an adequate size of NPs for ocular administration with a sustainable releasing profile. The interaction studies of NPs-PGZ showed that within the formulation the drug remains linked to the polymer. Regarding effectiveness, it was found that these systems decrease the level of inflammation in rabbit eyes with an optimum ocular tolerance. In conclusion, PGZ-NPs showed to be suitable systems to possible treatment of inflammatory ocular diseases. Acknowledgements: Authors would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel) Brazil and the Spanish Ministry of Science and Innovation grant (MAT2014-59134R) for financial support.
Dipali M.Dhoke
Rashtrasant Tukadoji Maharaj Nagpur University, India.
Title: A novel method to incorporate maleimide functional groups and HSA peptide on polymeric nanoparticles for hepatocyte-targeted delivery of lamivudine
Biography:
Dipali Dhoke is pursuing her Ph.D. from Department of Pharmaceutical Sciences, Rashtrasant Tukadokji Maharaj Nagpur University, Nagpur, India. She is awarded with a Rajiv Gandhi National fellowship from University Grant Commission for her Ph. D. work. Her Ph.D work focus on development and synthesis of polymeric nanoparticles with a targeting ligand for receptor specific delivery of some antiviral and anticancer drugs which increases the accumulation and retention of nanoparticles in the affected/diseased tissue.
Abstract:
Targeted delivery of a wide variety of payloads can be achieved using nanoparticles formulated using poly(D,L-lactide-co-glycolide)PLGA as a copolymer which has emerged as a most promising nanocarrier. However, PLGA nanoparticles (NPs) have limited types of functional groups available on the surface for conjugation to targeting ligands which is its important drawback. In this study the interfacial activity assisted surface functionalization (IAASF) technique can be used to incorporate reactive functional groups such as maleimide onto the surface of PLGA NPs. This maleimide group can be further linked with lactosaminated Human Serum Albumin (L-HSA) which acts as a ligand and target the asialoglycoprotein receptor (ASGPR) present in large amounts and with high affinity on hepatocytes. The ligand bound functionalized NPs were formulated by solvent evaporation method and optimized using Central Composite design. The incorporation of maleimide group and HSA was confirmed by FTIR, NMR, SDS-PAGE, in vitro cell uptake study and in vivo biodistribution study. The functionalized NPs were further characterized using dynamic light scattering, TEM, DSC and XRD. The encapsulation efficiency, in-vitro drug release behaviour and in vivo studies of drug-loaded-NPs were studied using ultra violet spectroscopy and HPLC methods. The functionalized NPs showed a burst release at beginning and sustained release until 24 h in physiological conditions. Functionalization of NPs with L-HSA peptide increased the cellular uptake of NPs 2-3-fold, and this enhancement in uptake was substantially reduced in the absence of the ligand. In-vivo biodistribution study suggested the enhanced target ability and accumulation of surface functionalized ligand bound NPs. In conclusion, the IAASF technique enabled the incorporation of reactive maleimide groups on PLGA NPs, which in turn permitted efficient conjugation of biologically active L-HSA peptide to the surface of PLGA NPs. Nucleoside analogs (NAs) like lamivudin when loaded in NPs conjugated with galactosyl terminating peptides selectively enter hepatocytes via the ASGPR and thus reduce the extrahepatic side effects of lamivudin in the treatment of chronic viral hepatitis.
Seongjae Jo
Yonsei University, Korea
Title: Copper II ions detection via Localized Surface Plasmon Resonance
Biography:
Seongjae Jo studied control and measurement theory at the Department of Control and Instrumentation Engineering at Korea University. Among various subjects, He was interested in sensors and went to graduate school to study nano and biosensors. He is currently studying for the 5th semester of graduate school as a master and doctor Integrated course. He is studying sensing methods using optics such as absorbance, Raman scattering, and fluorescence, and he is also studying the synthesis of gold nanoparticles for optical applications. His study is supported by the National Research Foundation of Korea (NRF)
under Grant no.NRF2015R1A1A1A05027581 and Korea University Future Research Grant.
Abstract:
As the modern technology has developed, the problem to toxicity of nano-scale materials continues to rise, so it has emerged as an important research project to detect the toxic agents. In particular, according to a recent study, small amounts of copper ions increase the growth rate of the tumor, so it is important to detect a low concentration of copper ions.
A conventional method for detecting ions is a use of inductively coupled plasma (ICP) that is expensive and has a hassle things like preprocess of the samples and stabilization of the plasma which is necessary. However, the use of localized surface plasmon resonance (LSPR) is one of the techniques using the optics which can easily and quickly detect materials on the substrate in real time. The substrate and chelators, antibodies, aptamers or ligands are conjugated, can bind the desired materials like ions, proteins, even enzymes and genes.
In this study, we made nanoplasmonic substrates using gold nanorods and D-penicillamine (DPA). D-penicillamine (DPA) is easy to conjugate with gold due to thiol group. But it is a chelator of the copper, so the DPA conjugated to the substrate is separated from the substrate and bonded to the copper II ions. Copper ion could be detected up to 100 picomolar (pM) concentration by using the nanoplasmonic substrate. It shows a unique selectivity for copper II ions. And we were able to detect copper in human blood-like environment. Based on this study, not only copper II ions but also other ions can be detected by making the substrate that can be more quickly and easily monitored for ions.
Agathe Figarol
University of Toulouse, France
Title: Polymeric self-assemblies for photodynamic therapy: a critical approach
Biography:
Agathe Figarol has developed her expertise in nano-bio interactions since her MSc. thesis in Health and the Environment (Cranfield University, UK). Looking at the cellular impact of silica nanomaterials on keratinocytes, she was already starting to combine the different approaches of physical chemistry and biology relying on her technical background in biology engineering (Université de Technologie de Compiègne, France). She further enhanced her pluridisciplinarity along her PhD exploring the impacts on physico-chemical characteristics of carbon nanotubes on macrophages (Ecole Nationale Supérieure des Mines de St-Etienne, France). Her confirmed enthusiasm for the dynamic and state-of-the-art field of nanobiology led her, after a year of discovery of the industrial strategy on toxicology, to join a collaborative project on polymeric nanovectors (IPBS and IMRCP laboratories from the Université Paul Sabatier de Toulouse, France).
Abstract:
Statement of the Problem: The work presented here suggests a new approach in the critical development of polymeric nanovectors for photodynamic therapy (PDT) against cancer. Whereas hundreds of studies quickly jump forward from formation of self-assemblies to biological application without having a thorough examination of the vector solution, we suggest having a parallel assessment of formation/characterization of the nanovectors and their biological activity. This is possible by first conducting a careful physical chemistry characterization of the vectors by both batch techniques (light and neutron scattering, electron microscopy, atomic force microscopy) and Asymmetrical Flow Field-Flow Fractionation (AsFlFFF) coupled to adequate detectors (refractometry, light scattering). This enables us to fully characterize the vectors regarding purity, size and zeta potential. Methodology & Theoretical Orientation: Data on both polymeric micelles and polymersomes are presented here, using`poly(ethyleneoxide-b-e-caprolactone), poly(ethyleneoxide-b-D,Llactide) and poly(ethyleneoxide-b-styrene). Self-assemblies exhibiting size range of 20-200 nm are presented and reveal the possible presence of different populations of nanovectors in some cases. Controlled mixtures of different nano-objects are also studied, as well as crosslinked systems. For each new vector, its ability to carry a photosensitizer (Pheophorbide a) for PDT is examined. The activity in PDT either in 2D and 3D cell culture is presented and compared on different batches, in link with the purity analysis. Here again, it becomes highly recommended to develop a critical approach considering in vitro analyses, since different efficiencies are clearly observed depending on the vectors and the 2D or 3D culture type.Conclusion & Significance: This work shows that selected mixtures of different vectors with different morphologies or sizes may lead to synergetic effects. Also, a strong influence of the crosslinking of thevector has been observed and will be presented (Figure 1).
Paola Castaldo
CEO of Fastissues srls. Naples, Italy.
Title: Modeling of 3D additive manufactured nanocomposite scaffolds

Biography:
Paola Castaldo is the CEO of Fastissues srls and she also is a high school teacher of Applied Mathematics. She earned a PhD in Mathematics for Economic and Financial Applications and a PhD in Technology and Production Engineering. Her skills are the result of years of experience in simulation, evaluation, teaching and administration both in research and education institutions.
Abstract:
Statement of the Problem: As bone tissue engineering is concerned, a scaffold has to provide a suitable mechanical function in order to withstand loads and to transfer the stress to the hosting tissue. Polymer-based based composite scaffolds can be processed through the additive manufacturing approach (e.g. FDM) in order to design custom made scaffolds. The purpose of this study is to analyze the stress distribution into a 3D nanocomposite scaffold according to a compressive state of stress.
Methodology & Theoretical Orientation: Cylindrical Polycaprolactone/nano-Hydroxyapatite 80/20w scaffolds were biomanufactured through a 3D plotter dispensing machine (Envisiontec GmbH, Gladbeck, Germany) at pressure of 8.5 bar and a temperature of about 120°C. Compression tests were carried out with an INSTRON 5566 at a rate of 1 mm/min. Finite element modelling (FEM) was carried out through the ANSYS software. The representative 3D scaffold design is shown in Figure 1A. The Young’s modulus and the Poisson’s ratio were set at 650MPa and 0.35, respectively. The mesh of a single unit of the cylindrical scaffold consisted of 397113 SOLID186 elements (Figure 1B).
Findings: As the scaffold undergoes a compression load, the compressive stress is mainly transferred through the regions where the plotted fibers cross each other and through part of the round boundary. Moreover, a tensile state of stress is clearly evident on the regions of fibers close to the crossing points (Figure 1C). FEM simulations are in a good agreement with the experimental results as suggested by Figures 1D.
Conclusion & Significance: FEM simulation in conjunction with experimental testing represent a very powerful tool to design custom made scaffolds.
Mina Emamzadeh
UCL School of Pharmacy,UK
Title: Laser triggered release of gemcitabine from polymer coated gold nanoshells for pancreatic cancer
Biography:
Mina Emamzadeh (BSc, MRes) is PhD student in cancer nanomedicine at UCL School of Pharmacy, London. Mina’s research is focused on the development of therapeutic nanoparticle for cancer therapy. In the present project, she develops radically new therapeutic protocols that combine lasers and gold nanoparticles to direct drugs at the diseased sites of the body in a specific manner without damaging healthy tissue. She also has experience in dendrimer-based nanomedicine.
Abstract:
The unique combination of optical and chemical properties of gold nanoparticles (GNPs) renders them an appealing nano-scale platform for cancer therapeutics. In this project we focused on the development of a new generation of theranostic GNPs for cancer treatment by the co-delivery of anti-cancer drugs in concert with confined laser induced photothermal tumor ablation. We anticipate that the combinatorial photo-chemotherapeutic protocol will exhibit significantly higher apoptotic cell rates without damaging the non-irradiated healthy tissue areas.Gold nanoshells (GNSs) were synthesized with the capability to carry and deliver gemcitabine and exert synergistic photo-chemo-therapeutic properties. A protein repellent thiol capped poly (ethylene glycol) methacrylate polymer, with molecular weight of 15000 g/mol, was synthesized by radical addition fragmentation (RAFT) polymerization and used as a particle stabilizing polymeric shield. Significant levels of stability enhancement were achieved allowing for the co-functionalization of GNSs with gemcitabine (GEM) for applications in assays and drug carrier systems. GNSs mediated strong photothermal effect owing to their strong surface plasmon absorption in the NIR region. This property was exploited for the controlled release of GEM using NIR light as the external photostimulus to trigger drug release. The drug loaded GNSs exhibited synergistic cytotoxicity against a model pancreatic cell line (MiaPaCa-2) owing to the concerted antitumor activity of GEM with the photothermal effect of the GNSs upon irradiation with the laser.
